Abstract
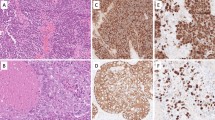
Fifteen midgut carcinoid tumors and 5 solid carcinomas of the intestine with carcinoid-like morphological features were evaluated histochemically and immunohistochemically with respect to various endocrine markers and expression of mutant p53 protein. Direct sequencing of the p53 gene after PCR amplification was carried out on microdissected cells from all tumors. All investigated carcinoid tumors showed chromogranin and argentaffin reaction, but lacked nuclear immunostaining with p53 antibodies. In 14 immunohistochemically negative midgut carcinoid tumors, no mutations were identified. One carcinoid tumor devoid of p53 staining was, however, found to contain mutation in exon 6 of the p53 gene. In contrast, the solid carcinomas were essentially chromogranin negative but all displayed clearly positive p53 staining in a variable number of cell nuclei. Sequence analysis of exons 5–8 of the p53 gene in the 5 carcinomas showed mutations in exons 6 and 7 in 2 tumors and in exon 8 in 2 other tumors, while no mutation was detected in the fifth tumor. The carcinoid tumors and the solid carcinomas of the small intestine are thought to derive histogenetically from endocrine cells and enterocytes, respectively. The present results substantiate that divergent mechanisms operate in the development of the two tumor types.
Similar content being viewed by others
References
Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, Bigner SH, Davidson N, Baylin S, Devilee P, Glover T, Collins FS, Weston A, Modali R, Harris CC, Vogelstein B. Mutations in the p53 gene occur in diverse human tumour types. Nature 342:705–708, 1989.
Holstein M, Sidransky D, Vogelstein B, Harris C. p53 mutations in human cancers. Science 253:49–53, 1991.
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res 51:6304–6311, 1991.
Harris C, Holstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med 329:1318–1327, 1993.
Grimelius L. A silver nitrate stain for alpha 2-cells in human pancreatic islets. Acta Soc Med Upsal 73:243–270, 1968.
Singh I. A modification of the Masson-Hamperl method for staining of argentaffin cells. Anat Anz 114:81,82, 1964.
Vojtesek B, Bartek J, Midgley CA, Lane DP. An immunohistochemical analysis of human nuclear phosphoprotein p53: new monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods 151:237–244, 1992.
Migdley CA, Fisher CJ, Bartek J, Vojtesek B, Lane DP, Barnes DM. Analysis of p53 expression in human tumours: an antibody raised against human p53 expressed inE. coli. J Cell Sci 101:183–189, 1992.
Hedrum A, Pontén F, Ren Z, Lundeberg J, Pontén J, Uhlén M. Sequence-based analysis of the human p53 gene based on mirodis-section of tumor biopsy samples. Biotechniques 17:118–129, 1994.
Berg C, Hedrum A, Holmberg A, Pontén F, Uhlén M, Lundberg J. Direct solid-phase sequence analysis of the human p53 gene by use of multiplex polymerase chain reaction and alpha-thiotriphosphate nucleotides. Clin Chem 41:1461–1466, 1995.
Hultman T, Ståhl S, Hornes E, Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res 17:4937–4946, 1989.
Hultman T, Bergh S, Moks T, Uhlén M. Bidirectional solid-phase sequencing of in vitro-amplified plasmid DNA. Biotechniques 10:84–93, 1991.
Wilander E, Scheibenpflug L, Eriksson B, Öberg K. Diagnostic criteria of classical carcinoids. Acta Oncol (OSLO) 30:469–475, 1991.
Barton CM, Staddon SL, Hughes CM, Hall PA, O'Sullivan C, Klöppel G, Theis B, Russel RC, Neoptolemos J, Williamson RC, Laine DP, Lemoine NR. Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer. Br J Cancer 64:1076–1082, 1991.
Scarpa A, Capelli P, Mukai K, Zamboni G, Oda T, Iacono C, Hirohashi S. Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol 142:1534–1543, 1993.
van den Berg F, Baas I, Polak M, Offerhaus J. Detection of p53 overexpression in routinely paraffin-embedded tissue of human carcinomas using a novel target unmasking fluid. Am J Pathol 142:381–385, 1993.
Bosari S, Viale G, Bossi P, Maggioni M, Coggi G, Murray JJ, Lee AK. Cytoplasmic accumulation of p53 protein: an independent prognostic indicator in colorectal adenocarcinomas. J Natl Cancer Inst 86:681–687, 1994.
Ramael M, Lemmens G, Eerdekens C, Buysse C, Deblier I, Jacobs W, van Marck E. Immunoreactivity for p53 protein in malignant mesothelioma and non-neoplastic mesothelium. J Pathol 168:371–375, 1992.
Hibi K, Kondo K, Akiyama S, Ito K, Takagi H. Frequent genetic instability in small intestinal carcinomas. Jpn J Cancer Res 86:357–360, 1995.
Lohmann DR, Funk A, Niedermeyer HP, Häupel S, Höfler H. Identification of p53 gene mutations in gastrointestinal and pancreatic carcinoids by nonradioisotopic SSCA. Virchows Arch B Cell Pathol 64:293–296, 1993.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weckström, P., Hedrum, A., Makridis, C. et al. Midgut carcinoids and solid carcinomas of the intestine: Differences in endocrine markers and p53 mutations. Endocr Pathol 7, 273–279 (1996). https://doi.org/10.1007/BF02739834
Issue Date:
DOI: https://doi.org/10.1007/BF02739834