Abstract
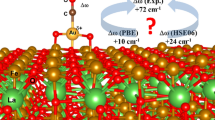
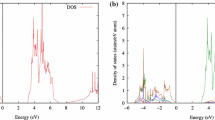
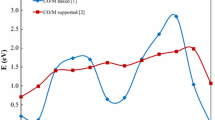
The adsorption properties of Au, Ag and Cu on TiO2 (110) rutile surfaces are examined using density functional theory slab calculations within the generalized gradient approximation. We consider five and four different adsorption sites for the metal adsorption on the stoichiometric and reduced surfaces, respectively. The metal-oxide bonding mechanism and the reactivity of metal atoms are also discussed based on the analyses of local density of states and charge density differences. This study predicts that Au atoms prefer to adsorb at the fourfold hollow site over the fivefold-coordinated Ti(5c) and in-plane and bridging O(2c) atoms with the adsorption energy of ≈0.6 eV. At this site, it appears that the covalent and ionic interactions with the Ti(5c) and the O(2c), respectively, contribute synergistically to the Au adsorption. At a neutral F 0s center on the reduced surface, Au binds to the surface via a rather strong ionic interaction with surrounding sixfold-coordinated Ti(6c) atoms, and its binding energy is much larger than to the stoichiometric surface. On the other hand, Ag and Cu strongly interact with the surface bridging O(2c) atoms, and the site between two bridging O(2c) atoms is predicted to be energetically the most favorable adsorption site. The adsorption energies of Ag and Cu at the B site are estimated to be ≈1.2 eV and ≈1.8 eV, respectively. Unlike Au, the interaction of Ag and Cu with a vacancy defect is much weaker than with the stoichiometric surface.
Similar content being viewed by others
References
Bates, S. P., Kresse, G. and Gillan, M. J., “A Systematic Study of the Surface Energetics and Structure of TiO2 (110) by First-principles Calculations,”Surf. Sci.,385, 386 (1997).
Bell, A. T., “The Impact of Nanoscience on Heterogeneous Catalysis,”Science,299, 1688 (2003).
Bennett, R. A., Stone, P., Price, N. J. and Bowker, M., “Two (1×2) Reconstructions of TiO2 (110): Surface Rearrangement and Reactivity Studied using Elevated Temperature Scanning Tunneling Microscopy,”Phys. Rev. Lett.,82, 3831 (1999).
Boccuzzi, F., Chiorino, A., Manzoli, M., Andreeva, D., Tabakova, T., Ilieva, L. and Iadakiev, V., “Cold, Silver and Copper Catalysts Supported OnTiO2 for Pure Hydrogen Production,”Catal. Today,75, 169 (2002).
Bogicevic, A. and Jennison, D. R., “Effect of Oxide Vacancies on Metal Island Nucleation,”Surf. Sci.,515, L481-L486 (2002).
Bredow, T. and Pacchioni, G., “Electronic Structure of an Isolated Oxygen Vacancy at the TiO2 (110) Surface,”Chem. Phys. Lett.,355, 417 (2002).
Campbell, C. T., Parker, S. C. and Starr, D. E., “The Effect of Size-Dependent Nanoparticle Energetics on Catalyst Sintering,”Science,298, 811 (2002).
Campbell, C. T., “Ultrathin Metal Films and Particles on Oxide Surfaces: Structural, Electronic and Chemisorptive Properties,”Surf. Sci. Rep.,27, 1 (1997).
Ceperley, D.M. and Alder, B. J., “Ground-state of the Electron Gas by a Stochastic Method,”Phys. Rev. Lett.,45, 566 (1980).
Charlton, G., Howes, P., Nicklin, C., Steadman, P., Taylor, J., Muryn, C., Harte, S., Mercer, J., McGrath, R., Norman, D., Turner, T. and Thornton, G., “Relaxation of TiO2(110)-(1×1) using Surface X-ray Diffraction,”Phys. Rev. Lett.,78, 495 (1997).
Choudhary, T.V. and Goodman, D.W., “Oxidation Catalyst by Supported Gold Nano-clusters,”Top. Catal.,21, 1 (2002).
Christensen, A. and Carter, E. A., “Adhesion of Ultrathin ZrO2(111) Films on Ni(111) from First Principles,”J. Chem. Phys.,114, 5816 (2001).
de Oliveira, A. L., Wolf, A. and Schuta, F., “Highly Selective Propene Epoxidation with Hydrogen/oxygen Mixtures over Titania-supported Silver Catalysts,”Catal. Lett.,73, 157 (2001).
Diebold, U., Anderson, J. F., Ng, K.-O. and Vanderbilt, D., “Evidence for the Tunneling Site on Transition-metal Oxides: TiO2 (110),”Phys. Rev. Lett.,77, 1322 (1996).
Eichler, A., Hafner, J., Furthmüller, J. and Kresse, G., “Structural and Electronic Properties of Rhodium Surfaces: An ab initio Approach,”Surf. Sci.,346, 300 (1996).
Ferrari, A.M. and Pacchioni, G., “Electronic Structure of F and V Centers on the MgO Surface,”J. Phys. Chem.,99, 17010 (1995).
Giordano, L., Pacchioni, G., Bredow, T. and Sanz, J. F., “Cu, Ag, and Au Atoms Adsorbed on TiO2 (110): Cluster and Periodic Calculations,”Surf. Sci.,471, 21 (2001).
Guo, Q., Cocks, I. and Williams, E. M., “Surface Structure of (1×2) Reconstructed TiO2 (110) Studied using Electron Stimulated Desorption ion Angular Distribution,”Phys. Rev. Lett.,77, 3851 (1996).
Hammer, B. and NØrskov, J. K., “Electronic Factors Determining the Reactivity of Metal Surfaces,”Surf. Sci.,343, 211 (1995).
Hansen, P. L., Wagner, J. B., Helveg, S., Rostrup-Nielsen, J. R., Clausen, B. S. and Topsoe, H., “Atom-Resolved Imaging of Dynamic Shape Changes in Supported Copper Nanocrystals,”Science,295, 2053 (2002).
Harrison, N. M., Wang, X.G., Muscat, J. and Scheffler, M., “The Influence of Soft Vibrational Modes on our Understanding of Oxide Surface Structure,”Faraday Discussions,114, 305 (1999).
Haruta, M., “Size-and Support-dependency in the Catalysis of Gold,”Catal. Today,36, 153 (1997).
Hayash, T., Tanaka, K. and Haruta, M., “Selective Vapor-phase Epoxidation of Propylene over Au/TiO2 Catalysts in the Presence of Oxygen and Hydrogen,”J. Catal.,178, 566 (1998).
Kolmakov, A. and Goodman, D.W., “Imaging Gold Clusters on TiO2 (110) at Elevated Pressures and Temperatures,”Catal. Lett.,70, 93 (2000).
Kolmakov, A. and Goodman, D.W., “Scanning Tunneling Microscopy of Gold Clusters on TiO2 (110): CO Oxidation at Elevated Pressures,”Surf. Sci.,490, L 597-L601 (2001).
Kresse, G. and Hafner, J., “Ab-initio Molecular-dynamics for Liquidmetals,”Phys. Rev. B,47, RC558 (1993)
Kresse, G. and Furthmüller, J., “Efficient Iterative Schemes for ab initio Total-energy Calculations using a Plane-wave Basis Set,”Phys. Rev. B,54, 11169 (1996).
Kresse, G. and Hafner, J., “Norm-conserving and Ultra-soft Pseudopotentials First-row and Transition Elements,”J. Phys.: Condens. Matter,6, 8245 (1994).
Lindan, P. J. D., Harrison, N. M., Gillan, M. J. and White, J.A., “Firstprinciples Spin-polarized Calculations on the Reduced and Reconstructed TiO2 (110) Surface,”Phys. Rev. B,55, 15919 (1997).
Lopez, N. and NØrskov, J.K., “Theoretical Study of the Au/TiO2 (110) Interface,”Surf. Sci.,515, 175 (2002).
Mattsson, A. E. and Jennison, D. R., “Computing Accurate Surface Energies and the Importance of Electron Self-energy in Metal/metaloxide Adhesion,”Surf. Sci.,520, L611-L618 (2002).
Matveev, A.V., Neyman, K. M., Yudanov, I.V. and Rosch, N., “Adsorption of Transition Metal Atoms on Oxygen Vacancies and Regular Sites of the MgO(001) Surface,”Surf. Sci.,426, 123 (1999).
Murray, P.W., Condon, N.G. and Thornton, G., “Effect of Stoichiometry on the Structure of TiO2 (110),”Phys. Rev. B,51, 10989 (1995).
Muscat, J., Harrison, N. M. and Thorton, G., “First-principles Study of Potassium Adsorption on TiO2 Surfaces,”Phys. Rev. B,59, 2320 (1999).
Ng, K.-O. and Vanderbilt, D., “Structure and Apparent Topography of TiO2 (110) Surfaces,”Phys. Rev. B,56, 10544 (1997).
Onishi, H. and Iwasawa, Y., “Reconstruction of TiO2 (110) Surface-STM Study with Atomic Scale Resolution,”Surf. Sci.,313, L783-L789 (1994).
Pang, C. L., Haycock, S.A., Raza, H., Murray, P.W., Thornton, G., Gulesren, O., James, R. and Bullett, D.W., “Added Row Model of TiO2 (110) 1×2,”Phys. Rev. B,58, 1586 (1998).
Perdew, J. and Zunger, A., “Self Interaction Correction to Density-functional Approximations for Many-electron Systems,”Phys. Rev. B,23, 5048 (1981).
Perdew, J., Chevary, J., Vosko, S., Jackson, K., Pederson, M., Singh, D. and Fiolhais, C., “Atoms, Molecules, Solids, and Surfaces-Applications of the Generalized Gradient Approximation for Exchange and Correlation,”Phys. Rev. B,46, 6671 (1992).
Reinhardt, P. and Hess, B. A., “Electronic and Geometrical Structure of Rutile Surfaces,”Phys. Rev. B,50, 12015 (1994).
Santra, A. K. and Goodman, D.W., “Oxide-supported Metal Clusters: Models for Heterogeneous Catalysts,”J. Phys.: Condens. Matter,14, R31-R62 (2002).
Schaub, R., Wahlström, E., RØnnau, A., LÆgsgaard, E., Stensgaad, I. and Besenbacher, F., “Oxygen-mediated Diffusion of Oxygen Vacancies on the TiO2 (110) Surface,”Science,299, 377 (2003).
Siegel, D. J., Hector, L.G. and Adams, J. B., “Adhesion, Atomic Structure, and Bonding at the Al (111)/α-Al2O3 (0001) Interface: A First Principles Study,”Phys. Rev. B,65, 85415 (2002).
ThiÊn-Nga, L. and Paxon, A. T., “Electronic Structure of 5d Transition Metals Adsorbed on the Stoichiometric (110) Rutile Surface,”Phys. Rev. B,58, 13233 (1998).
Valden, M., Lai, X. and Goodman, D.W., “Onset of Catalytic Activity of Gold Clusters on Titania with the Appearance of Nonmetallic Properties,”Science,281, 1647 (1998).
Vanderbilt, D., “Soft Slef-consistent Pseudopotentials in a Generalized Eigenvalue Formalism,”Phys. Rev. B,41, 7892 (1990).
Verdozzi, C., Jennison, D. R., Schultz, P.A. and Sears, M. P., “Sapphire (0001) Surface, Clean and with d-Metal Overlayers,”Phys. Rev. Lett.,82, 799 (1999).
Vijay, A., Mills, G. and Meitu, H., “Adsorption of Gold on Stoichiometric and Reduced Rutile TiO2 (110) Surfaces,”J. Chem. Phys.,118, 6536 (2003).
Vinet, P., Ferrante, J., Smith, J. R. and Hose, J.H., “A Universal Equation of State for Solids,”J. Phys.: Condens. Matter,19, L467-L473 (1986).
Wahlström, E., Lopez, N., Schaub, R., Thostrup, P., RØnnau, A., Africh, C., LÆgsgaard, E., NØrskov, J.K. and Besenbacher, F., “Bonding of Gold Nanoclusters o Oxygen Vacancies on Rutile TiO2 (110),”Phys. Rev. Lett.,90, 26101 (2003).
Wang, Y. and Hwang, G. S., “Adsorption of Au Atoms on Stoichiometric and Reduced TiO2 (110) Rutile Surfaces: A First Principles Study,”Surf. Sci.,542, 72 (2003).
Yang, Z., Wu, R. and Goodman, D.W., “First-principles Study of the Adsorption of CO on TiO2(110),”Phys. Rev. B,61, 14066 (2000).
Zhou, J., Kang, Y. C. and Chen, D.A., “Controlling Island Size Distributions: A Comparison of Nickel and Copper Growth on TiO2 (110),”Surf. Sci.,537, L429-L434 (2003).
Zhukovskii, Y. F., Kotomin, E. A., Jacobs, P.W. M. and Stoneham, A.M., “Ab Initio Modeling of Metal Adhesion on Oxide Surfaces with Defects,”Phys. Rev. Lett.,84, 1256 (2000).
“Electronic Factors Determining the Reactivity of Metal Surfaces,”Surf. Sci.,343, 211 (1995).
Author information
Authors and Affiliations
Corresponding author
Additional information
°This paper is dedicated to Professor Hyun-Ku Rhee on the occasion of his retirement from Seoul National University.
Rights and permissions
About this article
Cite this article
Pillay, D., Wang, Y. & Hwang, G.S. A comparative theoretical study of Au, Ag and Cu adsorption on TiO2 (110) rutile surfaces. Korean J. Chem. Eng. 21, 537–547 (2004). https://doi.org/10.1007/BF02705445
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705445