Abstract
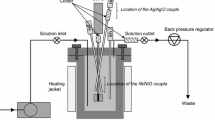
The kinetics of pyrite oxidation in sodium hydroxide solution were investigated in a stirred reactor, under temperatures ranging from 50 °C to 85 °C, oxygen partial pressures of up to 1 atm, particle size fractions from -150 + 106 to -38 + 10µm (-100 + 150 mesh to -400 mesh + 10 µ), and pH values of up to 12.5. The surface reaction is represented by the rate equation:-dN/dt = Sbk″pO0.5 2[oH- 0.25/(1 +k‴ pO2 0.5) where N represents moles of pyrite, S is the surface area of the solid particles,k″ andk″ are constants,b is a stoichiometric factor, pO2 is the oxygen partial pressure, and [OH-] is the hydroxyl ion concentration. The corresponding fractional conversion (X) vs time behavior follows the shrinking particle model for chemical reaction control: 1 - (1 -X)1/3 =k ct The rate increases with the reciprocal of particle size and has an activation energy of 55.6 kJ/mol (13.6 kcal/mol). The relationship between reaction rate and oxygen partial pressure resembles a Langmuir-type equation and thus suggests that the reaction involves adsorption or desorption of oxygen at the interface. The square-root rate law may be due to the adsorption of a dissociated oxygen molecule. The observed apparent reaction order with respect to the hydroxyl ion concentration is a result of a complex combination of processes involving the oxidation and nydrolysis of iron, oxidation and hydrolysis of sulfur, and the oxygen reduction.
Similar content being viewed by others
References
P.M.J. Gray:Trans. Inst. Min. Metall., 1955-56, vol. 65, pp. 55–65.
I.H. Warren:Aust. J. Appl. Sci., 1956, vol. 7, pp. 346–58.
J.F. Stenhouse and W.M. Armstrong:Can. Min. Met. Bull., 1952, Jan. 5, pp. 49–53.
J.T. Woodcock:The Aust. IMM, 1961, No. 198, pp. 47–84.
E.E. Smith and K.S. Shumate: No. 14010 FPS02/70, U.S. Department of Interior, Washington, DC, 1970.
H. Majima and E. Peters:TMS-AIME, 1966, vol. 236, pp. 1409–13.
M.B. Goldhaber:Am. J. Sci., 1983, vol. 238, pp. 193–217.
A.R. Burkin and A.M. Edwards:Proc. 6th Int. Cong. Mineral Processing, Cannes, 1963, A. Roberts, ed., Pergamon, New York, NY, 1965, pp. 159–69.
J.B. Hiskey and W.J. Schutt:Interfacing Technologies in Solution Mining, AIME, New York, NY, 1982, pp. 55–74.
T.D. Wheelock:Chern. Eng. Commun., 1980, vol. 12, pp. 137–59.
K.C. Chuang, M.C. Chen, R.T. Greer, R. Markuszewski, Y. Sun, and T.D. Wheelock:Chem. Eng. Commun., 1980, vol. 7, pp. 79–94.
M.A. McKibben and H.L. Barnes:Geochim. Cosmochim. Acta, 1986, vol. 50, pp. 1509–20.
C.T. Mathews and R.G. Robins:Aust. Chem. Eng., 1974, Nov.–Dec, pp. 19–24.
P.C. Singer and W. Stumm:Science, 1970, vol. 167, pp. 1121–23.
R.T. Lowson:Chem. Rev., 1982, vol. 82, pp. 461–97.
F.A. Forward and J. Halpern:J. Met. Trans. AIME, 1955, Mar., pp. 463–66.
V.H. Gottschalk and H.A. Buehler:Econ. Geol., 1912, vol. 7 (15), pp. 15–34.
D.R. McKay and J. Halpern:TMS-AIME, 1958, June, pp. 301–08.
L.K. Bailey and E. Peters:Can. Met. Q., 1976, vol. 15, pp. 333–44.
V.G. Papangelakis and G.P. Demopoulos:Hydrometallurgy, 1991, vol. 26, pp. 309–25.
T. Koslides and V.S.T. Ciminelli:Hydrometallurgy, 1992, vol. 30, pp. 87–106.
V.S.T. Ciminelli and K. Osseo-Asare:Metall. Mater. Trans. B, 1995, vol. 26B, pp. 209–18.
B.J. Heinrich, M.D. Grimes, and J. Puckett: inTreatise on Analytical Chemistry, I.M. Kolthoff and P.J. Elving, eds., Interscience, New York, NY, 1961, vol. 7, part II, section A, pp. 1–135.
V.S.T. Ciminelli: Ph.D. Thesis, The Pennsylvania State University, University Park, PA, 1987.
G.M. Kostina and A.S. Chernyak:Zhurnal Prikladnoi Khimii, 1979, vol. 52 (7), pp. 1532–35.
O. Levenspiel:Chemical Reaction Engineering, John Wiley and Sons, Inc., New York, NY, 1972.
Rate Processes in Extractive Metallurgy, H.Y. Sohn and M.E.Wadsworth, eds., Plenum Press, New York, NY, 1979.
J.J.C. Jansz:Hydrometallurgy, 1984, vol. 12, pp. 225–43.
R.E. Reed-Hill:Physical Metallurgy Principles, Van Nostrand,New York, NY, 1964.
P.G. Shewmon:Diffusion in Solids, McGraw-Hill, New York, NY, 1983.
CRC Handbook of Chemistry and Physics, 64th ed., R.C. Weast,ed., CRC Press, Boca Raton, FL, 1983.
Author information
Authors and Affiliations
Additional information
Formerly Graduate Student, Department of Mineral Engineering, Pennsylvania State University
Rights and permissions
About this article
Cite this article
Ciminelli, V.S.T., Osseo-Asare, K. Kinetics of pyrite oxidation in sodium hydroxide solutions. Metall Mater Trans B 26, 677–685 (1995). https://doi.org/10.1007/BF02651713
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02651713