Abstract
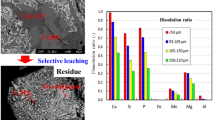
The reaction kinetics of phosphate ore particles reacting with excessive phosphoric acid was investigated. The effects of various operating parameters including reaction temperature (333.15–353.15 K), concentration of phosphoric acid (20–40 ωt% P2O5) and particle size (260, 190, 134, 96, 59 μm) on the reaction performance were investigated first. The results indicated that the reaction rate increased with the increase of reaction temperature, concentration of phosphoric acid, as well as the decrease of particle size. The Drozdov model could be used to describe the reaction behaviors. The activation energy results suggested that the rate-determining step was the diffusion of H+ through the residual layer. The precipitation of insoluble phosphates would cover on the surface and lead to a decrease of dissolution extent. Controlling the product forms is crucial to the improvement of dissolution extent. The specific surface area of the phosphate ore also had a significant impact on the reaction rate and dissolution extent.










Similar content being viewed by others
Data availability
There is no additional data available. All other data are available from the authors upon reasonable request.
References
Zarantoniello M, Pulido Rodriguez LF, Randazzo B, Cardinaletti G, Giorgini E, Belloni A, Secci G, Faccenda F, Pulcini D, Parisi G, Capoccioni F, Tibaldi E, Olivotto I (2022) Conventional feed additives or red claw crayfish meal and dried microbial biomass as feed supplement in fish meal-free diets for rainbow trout (Oncorhynchus mykiss): possible ameliorative effects on growth and gut health status. Aquaculture 554:738137
Vranješ B, Milićević D, Šefer D, Stefanović S, Ajtić J, Mitrović BM (2020) Presence of natural radionuclides and toxic elements in monocalcium phosphate, complete feed and pig manure. Sci Total Environ 720:137578
Lopez DA, Stein HH (2020) PSVI-8 Mineral composition of feed grade monocalcium phosphate. J Anim Sci 98:200
Prihanto A, Fitriyana DF, Muryanto S, Masykur I, Ismail R, Jamari J, Bayuseno AP (2021) Aqueous crystallization of monocalcium phosphate monohydrate with green mussel shells (Verna piridis) for calcium sources. J Environ Chem Eng 9:106913
Smol M, Kowalski Z, Makara A, Henclik A (2019) Comparative LCA study of different methods of the feed phosphates (FPs) production. J Cleaner Prod 239:117963
Zhou Y, Xiao C, Yang S, Yin H, Yang Z, Chi R (2021) Life cycle assessment of feed grade mono-dicalcium phosphate production in China, a case study. J Cleaner Prod 290:125182
Liu X, Wu F, Qu G, Jin C, Liu Y, Kuang L, Li H, Chen X, Wang Z, Cheng Y (2022) Application prospect of advanced oxidation technology in wet-process phosphoric acid production. J Environ Chem Eng 10:108868
Ma H, Feng X, Zeng B (2018) Self-anticorrosion for the combustion tower of heat recovered thermal process phosphoric acid production. Process Saf Environ Prot 118:330–347
Sun T, Li W, Xu F, Yu Z, Wang Z, Ouyang G, Xu D (2023) A new eco-friendly concrete made of high content phosphogypsum based aggregates and binder: mechanical properties and environmental benefits. J Cleaner Prod 400:136555
Bahsaine K, Mekhzoum MCM, Benzeid H, el kacem Qaiss A, Bouhfid R (2022) Recent progress in heavy metals extraction from phosphoric acid: a short review. J Ind Eng Chem 115:120–134
Peng B, Ma Z, Zhu Y, Tang L (2022) Release and recovery of fluorine and iodine in the production and concentration of wet-process phosphoric acid from phosphate rock. Miner Eng 188:107843
Wu S, Zhao L, Wang L, Huang X, Zhang Y, Feng Z, Cui D (2019) Precipitation-dissolution behaviors of rare earth ions in H3PO4-Ca(H2PO4)2 solutions. J Rare Earths 37:520–527
Nazarbekova S, Nazarbek U, Abdurazova P, Raiymbekov Y (2022) Study of solubility in the systems CaO-P2O5-SO3-H2O and CaO-P2O5-H2O on the example of sulfuric acid and phosphoric acid decomposition of phosphoric slag. AIP Conference Proceedings
Bakher Z, Kaddami M (2018) Thermodynamic equilibrium in the system H2O+P2O5+CaCO3 at 25 and 70 °C: application for synthesis of calcium phosphate products based on calcium carbonate decomposition. Fluid Phase Equilib 456:46–56
Bakher Z, Kaddami M (2018) Solubility study at high phosphorus pentoxide concentration in ternary system CaCO3+P2O5+H2O at 25, 35 and 70 °C. Fluid Phase Equilib 478:90–99
Mgaidi A, Mokni H (2018) Mathematical modeling of the dissolution of phosphate rock into various acidic medium. Hydrometallurgy 182:27–31
Serdyuk VV, Panov VP, Tereshchenko LY, Chekreneva GM (1982) Mechanism of the dissolution of apatite by phosphoric acid in the presence of electrolytes. Russ J Appl Chem 55:2004–2008
Huffman EO, Cate WE, Deming ME, Elmore KL (1957) Solubilities of phosphates-rates of solution of calcium phosphates in phosphoric acid solutions. J Agric Food Chem 5:266–275
Vandersluis S, Meszaros Y, Marchee WGJ, Wesselingh HA, Vanrosmalen GM (1987) The digestion of phosphate ore in phosphoric acid. Ind Eng Chem Res 26:2501–2505
Chaabouni A, Chtara C, Nzihou A, El Feki H (2013) Kinetic study of the dissolution of Tunisian natural phosphate or francolite in industrial phosphoric acid. J Adv Chem 6:908–916
Bandara AMTS, Senanayake G (2019) Dissolution of calcium, phosphate, fluoride and rare earth elements (REEs) from a disc of natural fluorapatite mineral (FAP) in perchloric, hydrochloric, nitric, sulphuric and phosphoric acid solutions: a kinetic model and comparative batch leaching of major and minor elements from FAP and RE-FAP concentrate. Hydrometallurgy 184:218–236
Gharabaghi M, Irannajad M, Noaparast M (2010) A review of the beneficiation of calcareous phosphate ores using organic acid leaching. Hydrometallurgy 103:96–107
Filippov LO, Filippova IV, Kaba OB, Fornasiero D (2021) In-situ study of the kinetics of phosphoric acid interaction with calcite and fluorapatite by Raman spectroscopy and flotation. Miner Eng 162:106729
Labgairi K, Jourani A, Kaddami M (2016) The ternary system H3PO4-Ca(OH)2–H2O isotherms at 15 and 45 °C. J Appl Solution Chem Model 5:5
Zhang S, Chen Y, Zhang T, Lv L, Zheng D, Zhong B, Tang S (2020) Separation of H3PO4 from HCl-wet-processing phosphate rocks leach liquor by TBP: extraction equilibria and mechanism study. Sep Purif Technol 249:117156
Štepec D, Tavčar G, Ponikvar-Svet M (2019) Measurement uncertainty evaluation and traceability assurance for total fluorine determination in vegetation by fluoride ion selective electrode. J Fluorine Chem 217:22–28
Zhang T, Lu Y, Luo G (2017) Effects of temperature and phosphoric acid addition on the solubility of iron phosphate dihydrate in aqueous solutions. Chin J Chem Eng 25:211–215
Kouzbour S, Gourich B, Gros F, Vial C, Stiriba Y (2022) Purification of industrial wet phosphoric acid solution by sulfide precipitation in batch and continuous modes: performance analysis, kinetic modeling, and precipitate characterization. J Cleaner Prod 380:135072
Senanayake G, Jayasekera S, Bandara AMTS, Koenigsberger E, Koenigsberger L, Kyle J (2016) Rare earth metal ion solubility in sulphate-phosphate solutions of pH range −0.5 to 5.0 relevant to processing fluorapatite rich concentrates: effect of calcium, aluminium, iron and sodium ions and temperature up to 80 °C. Miner Eng 98:169–176
Raza N, Zafar ZI, Kumar R (2015) Leaching of natural magnesite ore in succinic acid solutions. Int J Miner Process 139:25–30
Brahim K, Soussi-Baatout A, Khattech I, Jemal M (2017) Dissolution kinetics of fluorapatite in the hydrochloric acid solution. J Therm Anal Calorim 129:701–708
Levenspiel O (1998) Chemical reaction engineering. New York
Aly H, Ali M, Taha M, Rityi A, Maddi E (2013) Dissolution kinetics of western Desert phosphate rocks, Abu Tartur with hydrochloric acid. Arab J Nuclear Sci Appl 46:1–16
Acknowledgements
The authors gratefully acknowledge the financial aid from the National Key R&D Program of China (No. 2022YFC2904704).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, Q., Zhang, T., Lv, L. et al. Dissolution kinetics of phosphate ore particles in excessive phosphoric acid. Reac Kinet Mech Cat 136, 1211–1227 (2023). https://doi.org/10.1007/s11144-023-02413-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02413-z