Abstract
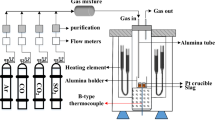
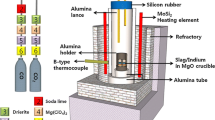
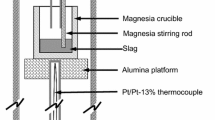
A gas-slag-metal equilibration technique was used to determine the sulfide capacity of the BaO-BaF2 system at 1473 and 1573 K. The dependence of carbonate capacity on the slag composition was also measured at these temperatures. It was found that the BaO-BaF2 system has the highest sulfide capacities among the fluxes which are of metallurgical interest. The dependence of sulfide and carbonate capacities on the partial pressure of O2 and CO2 was also investigated. The partial pressure of CO2 proved to have a strong effect on these values at the investigated temperatures. The influence of temperature on the sulfide and carbonate capacities was studied in the temperature range between 1423 and 1623 K. The data for sulfide and carbonate capacities were correlated in order to check if the carbonate capacity can be used as a measure of basicity of slags. It was found that the carbonate capacity can be used as a representative measure of the slag basicity at low contents of BaO and at temperatures higher than 1623 K when the carbonate dissolution into the slag is low and the ratio of the activity coefficient of a sulfide ion to that of a carbonate ion is independent of slag composition.
Similar content being viewed by others
References
S. Tabuchi and N. Sano:Tetsu-to-Hagané, 1985, vol. 71, pp. 687–92.
T. Hara, F. Tsukihashi, and N. Sano:Tetsu-to-Hagané, 1990, vol. 76, pp. 352–59.
M. Iwase, H. Fujiwara, E. Ichise, K. Ashida, and H. Akizuki:Iron and Steelmaker, 1988, vol. 15 (5), pp. 69–80.
T. Kawahara, K. Yamagata, and N. Sano:Steel Research, 1986, vol. 57 (4), pp. 160–65.
T. Kawahara, S. Shibata, and N. Sano:5th Int. Iron and Steel Congress, Process Technology Proc., Washington, DC, Iron and Steel Society, Inc., Warrendale, PA, 1986, vol. 6, pp. 691-96.
M. Iwase, H. Iritani, E. Ichise, and K. Shibata:Iron and Steelmaker, 1989, vol. 16 (3), pp. 67–71.
Carl Wagner:Metall. Trans. B, 1975, vol. 6B, pp. 405–09.
C.J.B. Fincham and F.D. Richardson:Proc. Roy. Soc, 1954, vol. A223, pp. 40–62.
M. Uo, F. Tsukihashi, and N. Sano:Steel Research, 1989, vol. 60 (11), pp. 496–502.
N. Fukatsu and Z. Kozuka:J. Jpn. Inst. Met., 1981, vol. 45, pp. 1263–70.
T. Rosenqvist:Trans. AIME, 1949, vol. 185, pp. 451–60.
E.T. Turkdogan:Physical Chemistry of High Temperature Technology, Academic Press, New York, NY, 1980.
E.T. Turkdogan:J. Iron Steel Inst., 1955, vol. 179, pp. 147–54.
B. Ozturk and E.T. Turkdogan:Met. Sci., 1984, vol. 18, pp. 299–309.
R.J. Hawkins, S.G. Meherali, and M.W. Davies:J. Iron Steel Inst., 1971, vol. 209, pp. 646–57.
R. Berryman, I.D. Sommerville, and F. Ishii:Iron and Steelmaker, 1987, vol. 14 (7), pp. 49–54.
Author information
Authors and Affiliations
Additional information
on leave from the Department of Metallurgy, Higher Institute of Chemical Technology, Sofia, Bulgaria
Rights and permissions
About this article
Cite this article
Rachev, I.P., Tsukihashi, F. & Sano, N. The thermodynamic behavior of sulfur in BaO-BaF2 slags. Metall Trans B 22, 333–338 (1991). https://doi.org/10.1007/BF02651232
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02651232