Abstract
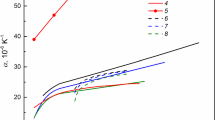
The thermodynamic properties of liquid Al-Mg alloys containing 0.027 to 95.50 at. Pct Mg were determined by measuring the emf, between 973 and 1073 K, of a magnesium concentration cell of the type Mg(l)|MgCl2-CaCl2 (eutectic melt, l)|Mg (in Al, l). Special attention has been given to low-magnesium aluminum alloys which are most commonly used in industry and for which definitive thermodynamic data are not reported in the literature. Alloys containing up to 12 at. Pct Mg follow Henry’s law, and the magnesium activity is given by the relation aMG = 0.88 XMG at 1073 K. Above 12 at. Pct Mg, the magnesium activity shows a small negative deviation from the ideal solution behavior. The activity of aluminum, however, closely follows the ideal solution behavior up to 75 at. Pct Mg and thereafter it shows a small negative deviation. The emf data have also been used to determine the free energy, entropy, enthalpy, and excess free energy for liquid Al-Mg alloys.
Similar content being viewed by others
References
E. E. Lukashenko and A. M. Pogodayev:Russian Metallurgy (Metally), 1971,(5), pp. 69–72.
M. M. Tsyplakova and K. L. Strelets:Journal of Applied Chemistry of the USSR, 1969, vol. 42 (11), pp. 2498–2503.
G. R. Belton and Y. K. Rao:Trans. TMS-AIME, 1969, vol. 245 (10), pp. 2189–93.
B.L. Tiwari and B.J. Howie: “Electrochemical Probe for Measur- ing Magnesium Concentration in Molten Aluminum,” U.S. Patent No. 4601810, July 22, 1986.
B. L. Tiwari:J. Metals, 1982, vol. 34 (7), pp. 54–58.
L. S. Darken and R. W. Gurry:Physical Chemistry of Metals, McGraw- Hill, New York, NY, 1953, p. 264.
R. Hultgren, R. L. Orr, P. D. Anderson, and K. K. Kelley:Selected Values of Thermodynamic Properties of Metals and Alloys, John Wiley and Sons, New York, NY, 1963.
R. A. Sharma:J. Chem. Thermodynamics, 1970, vol. 2, pp. 373–89.
M. Hansen:Constitution of Binary Alloys, 2nd ed., McGraw-Hill, New York, NY, 1958, p. 106.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tiwari, B.L. Thermodynamic properties of liquid Al-Mg alloys measured by the Emf method. Metall Trans A 18, 1645–1651 (1987). https://doi.org/10.1007/BF02646148
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02646148