Abstract
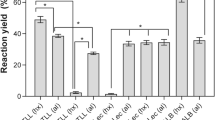
The abilities of four commercially available lipolytic enzymes [three immobilized lipases—Lipozyme IM-20, SP-435 (Novo Biolabs, Danbury, CT), and AY-30/Celite (Amano Enzyme Co., Ltd., Troy, VA)—and a nonimmobilized Amano phospholipase B preparation] to hydrolyze mixtures of triacylglycerols (TG) and phospholipids (PL) were determined. All of the lipases hydrolyzed both types of substrates in water, with maximum rates of TG hydrolysis exceeding those of PL hydrolysis by between 20- and 200-fold. The phospholipase B preparation was inactive against both TG and PL in water. All the enzymes showed some activity against lipids in hexane. The amount of activity was sharply dependent on the amount of water added to the reaction. Lipozyme IM-20 and AY-30/Celite hydrolyzed both TG and PL in hexane. Their estimated initial activities were between 10- and 100-fold lower than those in water. Complete hydrolysis of the TG (measured as the hydrolysis of at least one ester bond in each molecule) was achieved, whereas only 40–60% of the phosphatidylcholine (PC) and phosphatidylethanolamine (PE) were hydrolyzed. Lipase SP-435 was inactive against TG in hexane but hydrolyzed PC at a rate comparable to that seen in water, and it achieved complete hydrolysis of this substrate. Amano phospholipase B was inactive against TG in hexane but completely hydrolyzed the PC. The abilities of the enzymes to hydrolyze the TG, PC, and PE components of soybean soapstock, a by-product of edible oil production, were also examined. Lipozyme IM-20 hydrolyzed all the TG and a fraction of the PL in soapstock. SP-435 and AY-30/Celite were active only on soapstock that had been acidified prior to being dissolved in hexane. SP-435 displayed significant activity only toward PE under these conditions, whereas AY-30/Celite was active only toward TG. Phospholipase B was inactive against soapstock in hexane. The identity of the acid used in the acidification of soapstock affected the degree of hydrolysis by AY-30/Celite, with nitric and hydrochloric acids giving the best activity.
Similar content being viewed by others
References
Brockerhoff, H., and R.G. Jensen,Lipolytic Enzymes, Academic Press, New York, 1974.
Borgstrom, B., and H.L. Brockman (eds.),Lipases, Elsevier, New York, 1984.
Alberghina, L., R.D. Schmid and R. Verger (eds.),Lipases: Structure, Mechanism and Genetic Engineering, GBF Monographs, Vol. 16, VCH Publishers, New York, 1991.
Zaks, A., and A. Klibanov,Proc. Natl. Acad. Sci. USA 82:3192 (1985).
Dordick, J.S., inApplied Biocatalysis, Vol. 1, edited by H.W. Blanch, and D.S. Clark, Marcel Dekker, Inc., New York, 1991, pp. 1–51.
Zaks, A., inBiocatalysts for Industry, edited by J.S. Dordick, Plenum, New York, 1991, pp. 161–180.
Laboureur, P., and M. Labrousse,Compt. Rend. 259:4394 (1964).
de Haas, G.H., L. Sarda and J. Roger,Biochem. Biophys. Acta 106:638 (1965).
Slotboom, A.J., G.H. de Haas, P.P.M. Bonsen, G.J. Burbach-Westerhuis and L.L.M. Van Beenen,Chem. Phys. Lipids 4:15 (1970).
Shirai, K., R.L. Barnhart and R.L. Jackson,Biochem. Biophys. Research Commun. 100:591 (1981).
Van Oort, M.G., A.M.Th.J. Deveer, R. Dijkman, M. Tjeenk, H.M. Verheij, G.H. deHaas, E. Wenkzig and F. Gotz,Biochemistry 28:9278 (1989).
Enholm, C., W. Shaw, H. Greten and W.V. Brown,J. Biol. Chem. 250:6756 (1975).
Jensen, G.L., B. Daggy and A. Bensadoun,Biochim. Biophys. Acta 710:464 (1982).
Slotboom, A.J., H.M. Verheij and G.H. de Haas,Chem. Phys. Lipids 11:295 (1973).
Cox, J.W.,Fed. Proc. 36:852 (1977).
Hirashima, Y., A.A. Farooqui, E.J. Murphy and L.A. Horrocks,Lipids 25:344 (1990).
Van Middlesworth, F., M. Lopez, M. Zweerink, A.M. Edison and K. Wilson,J. Org. Chem. 57:4753 (1992).
Sarney, D.B., G. Fregapane and E.N. Vulfson,J. Am. Oil Chem. Soc. 71:93 (1994).
Yagi, T., T. Nakanishi, Y. Yoshizawa and F. Fukui,J. Ferment. Bioeng. 69:23 (1990).
Svensson, I., P. Adlercreutz and B. Mattiasson,Appl. Microbiol. Biotechnol. 33:255(1990).
Svensson, I., P. Adlercreutz and B. Mattiasson,J. Am. Oil Chem. Soc. 69:986 (1992).
Brockerhoff, H., P.C. Schmidt, J.W. Fong and L.J. Tirri,Lipids 11:421 (1976).
Yoshimoto, T., M. Nakata, S. Yamaguchi, T. Funada, Y. Saito and Y. Inada,Biotechnol. Lett. 8:771 (1986).
Mutua, L.N., and C.C. Akoh,J. Am. Oil Chem. Soc. 70:125 (1993).
Totani, Y., and S. Hara, Ibid.:848 (1991).
Haas, M.J., D.J. Cichowicz, J. Phillips and R. Moreau, Ibid.:111 (1993).
Haas, M.J., K. Scott, W. Jun and G. Janssen, Ibid.:483 (1994).
Snyder, H.E., and T.W. Kwon (eds.),Soybean Utilization, Van Nostrand Reinhold Co., New York, 1987, pp. 110–112.
Haas, M.J., D.J. Cichowicz and D.G. Bailey,Lipids 27:571 (1992).
Moreau, R.A., P.T. Asmann and H.A. Norman,Phytochemistry 29:2461 (1990).
Brady, L., A.M. Brzozowski, Z.S. Derewenda, E. Dodson, G. Dodson, S. Tolley, J.P. Turkenburg, L. Christiansen, B. Huge-Jensen, L. Norskov, L. Thim and U. Menge,Nature 343:767 (1990).
Grochulski, P., L. Yunge, J.D. Schrag, F. Bouthillier, P. Smith, D. Harrison, B. Rubin and M. Cygler,J. Biol. Chem. 268:12843 (1993).
Uppenberg, J., M.T. Hansen, S. Patkar and T.A. Jones,Structure 2:293 (1994).
Brzozowski, A.M., U. Derewenda, Z.S. Derewenda, G.G. Dodson, D.M. Lawson, J.P. Turkenburg, F. Bjorkling, B. Huge-Jensen, S.A. Patkar and L. Thim,Nature 351:491 (1991).
Derewenda, U., A.M. Brzozowski, D.M. Lawson and Z.S. Derewenda,Biochemistry 31:1532 (1992).
Author information
Authors and Affiliations
About this article
Cite this article
Haas, M.J., Cichowicz, D.J., Jun, W. et al. The enzymatic hydrolysis of triglyceride-phospholipid mixtures in an organic solvent. J Am Oil Chem Soc 72, 519–525 (1995). https://doi.org/10.1007/BF02638851
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02638851