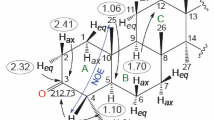
Abstract
The addition of N,N-dichlorourethan (DCU) to internal olefins has been found to be an acceptable reaction leading to the preparation of internal fatty acid aziridines. The yields of isolated epimino derivatives are in the 50–60% range, only slightly lower than those obtained by the iodine isocyanate (INCO) method. In order to compare the stereochemistry of the two methods, an IR spectroscopic procedure was developed which permits an estimation of the relative amounts ofcis- andtrans-aziridines in mixtures of the two. In agreement with literature reports, the INCO method is stereospecific, thecis-olefins giving rise tocis-aziridines and thetrans-olefins formingtrans-aziridines. On the other hand, DCU addition to olefins results in mixtures ofcis- andtrans-aziridines in which the latter isomers predominate. This finding is compatible with the reported free-radical nature of the DCU reaction.
Similar content being viewed by others
References
Fanta, P. E., in Heterocyclic Compounds with Three and Four Membered Rings, Part I, Edited by A. Weissberger, ed., Interscience Publishers, Inc., New York, 1964, p. 524–575 and references listed therein.
Gembitskii, P. A., N. M. Loim and D. S. Zhuk, Russ. Chem. Rev. (English Transl.)35, 105–122 (1966).
Gebelein, C. G., G. Swift and D. Swern, J. Org. Chem.32, 3314–3317 (1967).
McGhie, B. F., and B. T. Warren, Chem. and Ind.1968, 253.
Foglia, T. A., and D. Swern, J. Org. Chem.31, 3625–3631 (1966).
Foglia, T. A., and D. Swern, Ibid.32, 75–78 (1967).
Foglia, T. A., and D. Swern, Ibid.33, 766–771 (1968).
Dyen, M. E., H. C. Hamann and D. Swern, JAOCS43, 431–432 (1966).
Maerker, G., E. T. haeberer, L. M. Gregory and T. A. Foglia, Anal. Chem.41, 1698–1700 (1969).
Rosen, S., and D. Swern, Ibid.38, 1392–1397 (1966).
Hassner, A., and C. C. Heathcock, Tetrahedron Lett.1963, 393–397.
Hassner, A., and C. C. Heathcock, Ibid. 1125–1130 (1964).
Hassner, A., and C. C. Heathcock, Tetrahedron20, 1037–1042 (1964).
Hassner, A., and C. C. Heathcock, J. Org. Chem.29, 3640–3645 (1964).
Maerker, G., E. T. Haeberer and T. A. Foglia, Chem. and Ind.1968, 1524–1525.
Rabourn, W. J., and W. L. Howard, J. Org. Chem.27, 1039–1041 (1962).
Author information
Authors and Affiliations
Additional information
Agricultural Research Service, USDA.
About this article
Cite this article
Foglia, T.A., Maerker, G. & Smith, G.R. Preparation of epiminostearates. Comparison of methods. J Am Oil Chem Soc 47, 384–388 (1970). https://doi.org/10.1007/BF02632470
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02632470