Summary
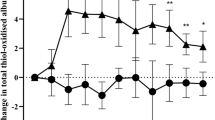
Glutathione status and products from lipid peroxidation [measured as thiobarbituric acid reactive substances (TBARS)] were determined in red and white muscle tissue of the rat. Marked differences between both muscle types were found in reduced glutathione (GSH) and oxidized glutathione (GSSG) content, exhibiting 163% and 183%, respectively, higher levels in red than in white muscle tissue, while the ratio of GSSG/GSH showed no differences. These characteristics may be due to an adaptive mechanism related to the 48% higher baseline level of TBARS in red muscle tissue. Immediately after 4 h of tourniquet-ischemia GSH, GSSG, and TBARS were increased (16%, 32%, 45% in white muscle; 19%, 49%, and 42% in red muscle, respectively), whereas the GSSG/GSH ratio remained unchanged. During the subsequent reperfusion period, GSH decreased within 2 h by 39% in white and 89% in red muscle to a minimal level of 5 mmol/g protein in both types of muscle. No recovery from the depletion was observed up to 12 h of reperfusion. The GSH decrease was parallelled by a marked increase of the GSSG/GSH ratio (150% in white and 450% in red muscle) and followed by about 150% increase in TBARS in both muscle types. This suggests that the increase in damaging TBARS is a secondary event after depletion of cellular antioxidants. Treatment of the animals during the reperfusion period with methyl-prednisolone, deferoxamine, or superoxide dismutase and catalase did not prevent the GSH decrease, but were effective in reducing the GSSG/GSH ratio to near normal and reducing the TBARS increase by about 50%.
Similar content being viewed by others
References
Baldwin KM, Klinkerfuss GH, Terjung RL. Molé PA, Holloszy JO (1972) Respiratory capacity of white, red, and intermediate muscle: adaptive response to exercise. Am J Physiol 222:373–378
Beutler E, West C (1977) Comment concerning a fluorometric assay for glutathione. Anal Biochem 81:458–460
Cadenas E, Brigelius R, Akerboom T, Sies H (1983) Oxygen radicals and hydroperoxides in mammalian organs: aspects of redox cycling and hydrogen peroxide metabolism. In: Sund H, Ullrich V (eds) Biological oxidations. Springer, Berlin Heidelberg New York, pp 288–309
Deneke SM, Fanburg BL (1989) Regulation of cellular glutathione. Am J Physiol 257: L163-L173
Feng LJ, Berger BE, Lysz TW, Shaw WW (1988) Vasoactive prostaglandines in the impending no-reflow state: evidence for a primary disturbance in microvascular tone. Plast Reconstruct Surg 81:755–765
Gorski J, Hood DA, Terjung RL (1986) Blood flow distribution in tissues of perfused rat hindlimb preparations. Am J Physiol 250:E441-E448
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Inauen W, Suzuki M, Granger DN (1989) Mechanisms of cellular injury: potential sources of oxygen free radicals in ischemia/reperfusion. Microcirc Endothelium Lymphatics 5:143–155
Lindsay T, Walker PM, Mickle DAG, Romaschin AD (1988) Measurement of hydroxy-conjugated diens after ischemia-reperfusion in canine skeletal muscle. Am J Physiol 254: H578–583
Lindsay T, Rosmaschin A, Walker PM (1989) Free radical mediated damage in skeletal muscle. Microcirc Endothelium Lymphatics 5:157–170
McKay RH, Higuchi DA, Winder WW, Fell RD, Brown BE (1983) Tissue effects of iron deficiency in the rat. Biochim Biophys Acta 757:352–358
McKelvey TG, Höllwarth ME, Ganger DN, Engerson TD, Landler U, Jones HP (1988) Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am J Physiol 254:G753-G760
Menger MD, Sack F-U, Barker JH, Feifel G, Meßmer K (1988) Quantitative analysis of microcirculatory disorders after prolonged ischemia in skeletal muscle. Res Exp Med 188: 151–156
Nicotera P, Orrenius S (1986) Thiols in the protection from biological reactive intermediates. Adv Exp Med Biol 197:41–51
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Salaris SC, Babbs CF (1988) A rapid, widely applicable screen for drugs that supress free radical formation in ischemia/reperfusion. J Pharmacol Methods 20:335–345
Salminen A, Vihko V (1983) Endurance training reduces the susceptibility of mouse skeletal muscle to lipid peroxidation in vitro. Acta Physiol Scand 117:109–113
Shan X, Aw TY, Jones DP (1990) Glutathione-dependent protection against oxidative injury. Pharmacol Ther 47:61–71
Sies H, Cadenas E (1983) Biological basis of detoxication of oxygen free radicals. In: Caldwell J, Jakoby WB (eds) Biological basis of detoxication. Academic Press. New York, pp 181–211
Smith JK, Carden DL, Korthuis EJ (1989) Role of xanthine oxidase in postischemic microvascular injury in skeletal muscle. Am J Physiol 257:H1782-H1789
Smith JK, Grisham MB, Granger DN, Korthuis RJ (1989) Free radical defense mechanisms and neutrophil infiltration in postischemic skeletal muscle. Am J Physiol 256:H789-H793
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke EK, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchinonic acid. Anal Biochem 150:76–85
Storck PE, Majno G (1969) Vascular response to experimental tourniquet ischemia. Surg Gynecol Obstet 129:309–318
Weiss SJ (1986) Oxygen, ischemia, and inflammation. Acta Physiol Scand Suppl 548:9–38
Yokota J, Minei JP, Fantint GA, Shires GT (1989) Role of leukocytes in reperfusion injury of skeletal muscle after partial ischemia. Am J Physiol 257:H1068-H1075
Ziegler DM (1985) Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem 54:305–329
Zimmerman BJ, Grisham MB, Granger DN (1990) Role of oxidants in ischemia/reperfusion-induced granulocyte infiltration. Am J Physiol 258:G185-G190
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Purucker, E., Egri, L., Hamar, H. et al. Differences in glutathione status and lipid peroxidation of red and white muscles: alterations following ischemia and reperfusion. Res. Exp. Med. 191, 209–217 (1991). https://doi.org/10.1007/BF02576676
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02576676