Abstract
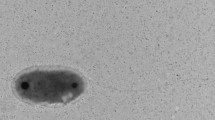
A gram-negative, non-motile, non-marine, nitrite-oxidizing bacterium was isolated from an enrichment culture initiated with a sample from a partially corroded area of an iron pipe of a heating system in Moscow, Russia. The cells were 0.9–2.2 μm×0.2–0.4 μm in size. They were helical- to vibroid-shaped and often formed spirals with up to three turns 0.8–1.0 μm in width. The organism possessed an enlarged periplasmic space and lacked intracytoplasmic membranes and carboxysomes. The cells tended to excrete extracellular polymers, forming aggregates. The bacterium grew optimally at 39°C and pH 7.6–8.0 in a mineral medium with nitrite as sole energy source and carbon dioxide as sole carbon source. The optimal nitrite concentration was 0.35 mM. Nitrite was oxidized to nitrate stoichiometrically. The doubling time was 12 h in a mineral medium with 7.5 mM nitrite. The cell yield was low; only 0.9 mg protein/l was formed during oxidation of 7.5 mM nitrite. Under anoxic conditions, hydrogen was used as electron donor with nitrate as electron acceptor. Organic matter (yeast extract, meat extract, peptone) supported neither mixotrophic nor heterotrophic growth. At concentrations as low as 0.75 g organic matter/l or higher, growth of nitrite-oxidizing cells was inhibited. The cells contained cytochromes of theb- andc-type. The G+C content of DNA was 56.9±0.4 mol%. The chemolithoautotrophic nitrite-oxidizer differed from the terrestrial members of the genusNitrobacter with regard to morphology and substrate range and equaledNitrospira marina in both characteristics. The isolated bacterium is designated as a new species of the genusNitrospira. Comparative analysis of 16S rRNA gene sequences revealed a moderate phylogenetic relationship toNitrospira marina, leptospirilla,Thermodesulfovibrio yellowstonii, “Magnetobacterium bavaricum”, and the isolate OPI-2. Initial evidence is given that these organisms represent a new phylum of the domain bacteria.
Similar content being viewed by others
References
Aggag M, Schlegel HG (1973) Studies on a gram-positive hydrogen bacterium,Nocardia opaca Strain 1b. Arch Microbiol 88:299–318
Bock E (1976) Growth ofNitrobacter in the presence of organic matter. 2. Chemoorganotrophic growth ofNitrobacter agilis. Arch Microbiol 108:305–312
Bock E, Heinrich G (1969) Morphologische Untersuchungen an den Zellen vonNitrobacter winogradskyi Buch. Arch Mikrobiol 69:149–159
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem 72:248–254
DeLey J (1970) Reexamination of the association between melting point, buoyant density and chemical base composition of desoxyribonucleic acid. J Bacteriol 101:733–754
Dupuy N, Willems A, Pot B, Dewettinck D, Vandenbruaene, Maestrojuan G, Dreyfus B, Kersters K, Collins MD, Gillis M (1994) Phenotypic and genotypic characterization of bradyrhizobia nodulating the leguminous treeAcacia albica. Int J Syst Bacteriol 44:461–473
Felsenstein J (1982) Numerical methods for inferring phylogenetic trees. Q Rev Biol 57:379–404
Freitag A, Rudert M, Bock E (1987) Growth ofNitrobacter by dissimilatory nitrate reduction. FEMS Microbiol Lett 48:105–109
Garcia-Horsman JA, Barquera B, Rumbley J, Ma J, Gennis RB (1994) The superfamily of heme-copper respiratory oxidases. J Bacteriol 176:5587–5600
Henry EA, Devereux R, Make JS, Gilmour CC, Woese CR, Mandelco L, Schauder R, Remsen CC, Mitchell R (1994) Characterization of a new thermophilic sulfate-reducing bacterium:Thermodesulfovibrio yellowstonii, gen. nov. sp. nov.: its phylogenetic relationship toThermodesulfobacterium commune and their origins deep within the bacterial domain. Arch Microbiol 161:62–69
Kalthoff H, Fehr S, Sundermeyer H, Renwrantz L, Bock E (1979) A comparison by means of antisera and lectins of surface structures ofNitrobacter winogradskyi andN. agilis. Curr Microbiol 2:375–380
Koops HP, Harms H (1985) Desoxyribonucleic acid homologies among 96 strains of ammonia-oxidizing bacteria. Arch Microbiol 141:214–218
Lane DJ, Harrison AP, Stahl DA, Pace B, Giovanni SJ, Olsen GJ, Pace NR (1992) Evolutionary relationship among sulfur- and iron-oxidizing bacteria. J Bacteriol 174:269–278
Larsen N, Olsen GJ, Maidak BL, McCaughey MJ, Overbeek R, Macke TJ, Marsh TL, Woese CR (1993) The ribosomal database project, Nucleic Acids Res 21:3021–3023
Lemberg R, Barrett J (1973) The cytochromes. Academic Press, New York
Ludwig W, Schleifer KH (1994) Bacterial phylogeny based on 16S and 23S rRNA sequence analyses. FEMS Microbiol Rev 15:155–173
Marmur Y (1961) A procedure for the isolation of desoxyribonucleic acid from microorganisms. J Mol Biol 3:208–218
Meincke M, Bock E, Kastrau D, Kroneck PMH (1992) Nitrite oxidoreductase fromNitrobacter hamburgensis: redox centers and their catalytic role. Arch Microbiol 158:127–131
Milde K, Bock E (1985) Comparative studies of membrane proteins ofNitrobacter hamburgensis andNitrobacter winogradskyi. FEMS Microbiol Lett 26:135–139
Murray RGE, Watson SW (1965) Structure ofNitrosocystis oceanus and comparison withNitrosomonas andNitrobacter. J Bacteriol 89:1594–1609
Neefs JM, Van de Peer Y, De Rijk P, Chapelle S, De Wachter R (1993) Compilation of small ribosomal subunit RNA structures. Nucleic Acids Res 21:3025–3049
Orso S, Gouy M, Navarro E, Normand P (1994) Molecular phylogenetic analysis ofNitrobacter spp. Int J Syst Bacteriol 44:83–86
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. Cell Biol 18:208–212
Rice CM, Fuchs R, Higgins DG, Stoehr PJ, Cameron GN (1993) The EMBL data library. Nucleic Acids Res 21:2967–2971
Smith AJ, Hoare DS (1968) Acetate assimilation byNitrobacter agilis in relation to its “obligate autotrophy”. J Bacteriol 95:844–855
Spector T (1978) Refinement of the Coomassie blue method of protein quantification. Anal Biochem 86:142–146
Spring S, Amann R, Ludwig W, Schleifer KH, Van Gemerden H, Petersen N (1993) Dominating role of an unusual magnetotactic bacterium in the microaerophilic zone of a freshwater sediment. Appl Environ Microbiol 59:2397–2403
Springer N, Ludwig W, Amann R, Schmidt HJ, Görtz HD, Schleifer KH (1993) Occurrence of fragmented 16S rRNA in an obligate bacterial endosymbiont ofParamecium caudatum. Proc Natl Acad Sci USA 90:9892–9895
Stackebrandt E, Ludwig W (1994) The importance of using outgroup reference organisms in phylogenetic studies: theAtopobium case. Syst Appl Microbiol 17:39–43
Steinmüller H, Bock E (1976) Growth ofNitrobacter in the presence of organic matter. 1. Mixotrophic growth. Arch Microbiol 108:299–304
Sundermeyer-Klinger H, Meyer W, Warninghoff B, Bock E (1984) Membrane-bound nitrite oxidoreductase ofNitrobacter: evidence for a nitrate reductase system. Arch Microbiol 140:153–158
Teske A, Alm E, Regan JM, Toze S, Rittmann BE, Stahl DA (1994) Evolutionary relationship among ammonia- and nitriteoxidizing bacteria. J Bacteriol 176:6623–6630
Watson ML (1958) Staining of tissue sections for electron microscopy with heavy metals. Biophys Biochem Cytol 4:475–478
Watson SW (1971) Taxonomic consideration of the familyNitrobacteriaceae Buchanan. Request for opinions. Int J Syst Bacteriol 21:254–270
Watson SW, Waterbury JB (1971) Characteristics of two marine nitrite-oxidizing bacteria,Nitrospina gracilis nov. gen. nov. spec. andNitrococcus mobilis nov. gen. nov. sp. Arch Mikrobiol 77:203–230
Watson SW, Bock E, Valois FW, Waterbury JB, Schlosser U (1986)Nitrospira marina gen. nov. sp. nov.: a chemolithoau-totrophic nitrite-oxidizing bacterium. Arch Microbiol 144:1–7
Willems A, Collins MD (1992) Evidence for a close genealogical relationship betweenAfipia (the causal organism of cat scratch disease),Bradyrhizobium japonicum andBlastobacter denitrificans. FEMS Microbiol Lett 75:241–246
Wong FYK, Stackebrandt E, Ladha JK, Fleischman DE, Date RA, Fuerst JA (1993) Phylogenetic analysis ofBradyrhizobium japonicum and photosynthetic stem-nodulating bacteria fromAeschynomene species grown in separated geographical regions. Appl Environ Microbiol 60:940–946
Yamanaka T, Kamita Y, Fukumori Y (1981) Molecular and enzymatic properties of cytochromeaa 3-type terminal oxidase derived fromNitrobacter agilis. J Biochem 89:265–273
Yanaga M, Yamasoto K (1993) Phylogenetic analysis of the family Rhizobiaceae and related bacteria by sequencing 16S rRNA gene using PCR and DNA sequencer. FEMS Microbiol Lett 107:115–120
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ehrich, S., Behrens, D., Lebedeva, E. et al. A new obligately chemolithoautotrophic, nitrite-oxidizing bacterium,Nitrospira moscoviensis sp. nov. and its phylogenetic relationship. Arch. Microbiol. 164, 16–23 (1995). https://doi.org/10.1007/BF02568729
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02568729