Abstract
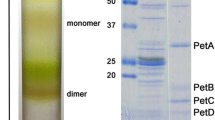
Nitrite oxidoreductase was isolated from mixotrophically grown cells of Nitrobacter hamburgensis. The enzyme purified from heat treated membranes was homogeneous by the criteria of polyacrylamide gel electrophoresis and size exclusion chromatography. The monomeric form consisted of two subunits with Mr 115000 and 65000, respectively. The dimeric form of the enzyme contained 0.70 molybdenum, 23.0 iron, 1.76 zinc, and 0.89 copper. The catalytically active enzyme was investigated by visible and electron paramagnetic resonance spectroscopy (EPR) under oxidizing (as isolated), reducing (dithionite), and turnover (nitrite) conditions. As isolated the enzyme exhibited a complex set of EPR signals between 5–75 K, originating from several ironsulfur and molybdenum (V) centers. Addition of the substrate nitrite, or the reducing agent dithionite resulted in a set of new resonances. The molybdenum and the iron-sulfur centers of nitrite oxidoreductase from Nitrobacter hamburgensis were involved in the transformation of nitrite to nitrate.
Similar content being viewed by others
Abbreviations
- EPR:
-
electron paramagnetic resonance
- ICP-AES:
-
inductively coupled plasma-atomic emission spectrometry
- NaPi :
-
sodium phosphate
- PAGE:
-
polyacrylamide gel electrophoresis
- SDS:
-
sodium dodecyl sulfate
References
Beuscher N, Mayer F, Gottschalk G (1974) Citrate lyase from Rhodopseudomonas gelationosa: purification, electron microscopy and subunit structure. Arch Microbiol 100: 307–328
Bock E, Sundermeyer-Klinger H, Stackebrandt E (1983) New facultative lithoautotrophic nitrite-oxidizing bacteria. Arch Microbiol 136: 281–284
Bock E, Koops H-P, Harms H (1989) Nitrifying bacteria. In: Schlegel HG, Bowien B (eds) Autotrophic bacteria. Springer Berlin Heidelberg New York, pp 81–96
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Cammack R, Weiner JH (1990) Electron paramagnetic resonance spectroscopic characterization of dimethyl sulfoxide reductase of Escherichia coli. Biochem 29: 8410–8416
Craig JA, Holm RH (1989) Reduction of nitrate to nitrite by molybdenum-mediated atom transfer: a nitrate reductase analogue reaction system J Am Chem Soc 111: 2111–2115
Fogo JK, Popowsky M (1949) Spectrophotometric determination of hydrogen sulfide. Methylene blue method. Anal Chem 21: 732–734
Fukuoka M, Fukumori Y, Yamanaka T (1987) Nitrobacter winogradskyi cytochrome a 1 c 1 is an iron-sulfur molybdo-enzyme having hemes a and c. J Biochem (Tokyo) 102: 525–530
Holm RH (1990) The biologically relevant oxygen atom transfer chemistry of molybdenum: from synthetic analogue systems to enzymes. Coord Chem Rev 100: 183–221
Hooper AB (1989) Biochemistry of the nitrifying lithoautotrophic bacteria. In: Schlegel HG, Bowien B (eds) Autotrophic bacteria. Springer Berlin Heidelberg New York, pp 239–265
Ingledew WJ, Halling PJ (1976) Paramagnetic centers of the nitrite oxidizing bacterium Nitrobacter. FEBS Lett 67: 90–93
Johnson MK, Benett DE; Morningstar JE, Adams MWW, Mortenson LE (1985) The iron-sulfur cluster composition of Escherichia coli nitrate reductase. J Biol Chem 260: 5456–5463
Kay CJ, Solomonson LP, Barber MJ (1990) Oxidation-reduction potentials of flavin and Mo-pterin centers in assimilatory nitrate reductase: variation with pH. Biochem 29: 10823–10828
Kessler DL, Rajagopalan KV (1972) Purification and properties of sulfite oxidase from chicken liver. J Biol Chem 247: 6566–6573
Krüger B, Meyer O, Nagel M, Andreesen JR, Meincke M, Bock E, Blümle S, Zumft WG (1987) Evidence for the presence of bactopterin in the eubacterial molybdoenzymes nicotinic acid dehydrogenase, nitrite oxidoreductase, and respiratory nitrate reductase. FEMS Microbiol Lett 48: 225–227
Meincke M (1990) Untersuchungen zur Struktur und Funktion von Redoxzentren im Enzym Nitrit-Oxidoreduktase. Dissertation Universität Hamburg
Milde K, Bock E (1984) Isolation and partial characterization of inner and outer membrane fractions of Nitrobacter hamburgensis. FEMS Microbiol Lett 21: 137–141
Renosto F, Ornitz DM, Peterson D, Segel IH (1981) Nitrate reductase from Penicillium chrysogenum. Purification and kinetic mechanism J Biol Chem 256: 8616–8625
Riester J, Zumft WG, Kroneck PMH (1989) Nitrous oxide reductase from Pseudomonas stutzeri. Redox properties and spectroscopic characterization of different forms of the multicopper enzyme. Eur J Biochem 178: 751–762
Solomonson LP, Barber MJ, Howard WD, Johnson JL, Rajagopalan KV (1984) Electron paramagnetic resonance studies on the molybdenum center of assimilatory NADH: nitrate reductase from Chlorella vulgaris. J Biol Chem 259: 849–853
Sundermeyer-Klinger H, Meyer W, Warninghoff B, Bock E (1984) Membrane-bound nitrite oxidoreductase of Nitrobacter: evidence for a nitrate reductase system. Arch Microbiol 140: 153–158
Tanaka Y, Fukumori Y, Yamanaka T (1983) Purification of cytochrome a 1 c 1 from Nitrobacter agilis and characterization of nitrite oxidation system of the bacterium. Arch Microbiol 135: 265–271
Vincent SP, Bray RC (1978) Electron-paramagnetic-resonance studies on nitrate reductase from Escherichia coli K12. Biochem J 171: 639–647
Yamanaka T, Kamita Y, Fukumori Y (1981) Molecular and enzymatic propertics of “cytochrome aa 3”-type terminal oxidase from Nitrobacter agilis. J Biochem (Tokyo) 89: 265–273
Yamanaka T, Fukumori Y (1988) The nitrite oxidizing system of Nitrobacter winogradskyi. FEMS Microbiol Rev 54: 259–270
Yamazaki T, Fukumori Y, Yamanaka T (1985) Cytochrome a 1 of Nitrosomonas europaea resembles aa 3-type cytochrome c oxidase in many respects. Biochim Biophys Acta 810: 174–183
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meincke, M., Bock, E., Kastrau, D. et al. Nitrite oxidoreductase from Nitrobacter hamburgensis: redox centers and their catalytic role. Arch. Microbiol. 158, 127–131 (1992). https://doi.org/10.1007/BF00245215
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00245215