Summary
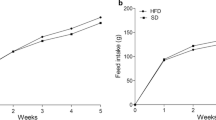
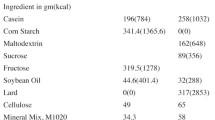
High fat-sucrose (HFS) diets can reportedly produce glucose intolerance and hyperinsulinemia that may indirectly have deleterious effects on bone. The effects of a high-fat diet on calcium absorption, bone calcium content, and bone mechanical properties, however, remain controversial. Thus, we examined the morphological and biomechanical adaptations in limb bones of rats that were fed a HFS diet. Female Sprague-Dawley rats (8 weeks old) were randomly assigned to two groups, either a control group (n=9) fed a standard diet (low-fat complex-carbohydrate) or an experimental group (n=9) fed a HFS diet for 10 weeks. The right tibia and second metatarsus (MT) were fractured in three-point bending, and contralateral bones were used for morphological and histological analyses. HFS tibias had significantly lower maximum load and failure energy, and tensile stress at the proportional limit for both HFS tibia and MT was significantly less than controls. In addition, the elastic modulus and density of the HFS MT was significantly lower than controls. Geometry of the tibial mid-diaphysial cross section did not differ for the two diets, but the cortical cross-sectional area of HFS MT increased significantly compared to control MT. The total number of osteons in the middiaphysis of HFS MT decreased, but tibial and MT porosities did not change with the HFS diet. Our results suggest that the deleterious effects of the HFS diet may be more related to changes in the material properties of the cortical bone rather than to osteoporotic changes in the bone.
Similar content being viewed by others
References
Grimditch GK, Barnard RJ, Sternlicht E, Whitson RH, Kaplan SA (1987) Effect of diet on insulin binding and glucose transport in rat sarcolemmal vesicles. Am J Physiol 252:E420-E425
Grimditch GK, Barnard RJ, Hendricks L, Weitzman D (1988) Peripheral insulin sensitivity as modified by diet and exercise training. Am J Clin Nutr 48:38–43
Lemann J Jr, Lennon EJ, Piering WR, Prien EL Jr, Ricanati ES (1970) Evidence that glucose ingestion inhibits net renal tubular reabsorption of calcium and magnesium in man. J Lab Clin Med 75:578–585
Wood RJ, Allen LH (1983) Evidence for insulin involvement in arginine- and glucose-induced hypercalciuria in the rat. J Nutr 113:1561–1567
DeFronzo RA, Cooke CR, Andes R, Faloona GR, Davis PJ (1975) The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 55:845–855
Holl MG, Allen LH (1987) Sucrose ingestion, insulin response and mineral metabolism in humans. J Nutr 117:1229–1233
Rosenbloom AL, Lezotte DC, Weber T, Gudat J, Heller DR, Weber ML, Klein S, Kennedy BB (1977) Diminution of bone mass in childhood diabetes. Diabetes 26:1052–1055
Levin ME, Boisseau VC, Avioli LV (1976) Effects of diabetes mellitus of bone mass in juvenile and adult onset diabetics. N Engl J Med 294:241–245
McNair P, Madsbad C, Christiansen C, Faber OK, Transbol I, Binder C (1978) Osteopenia of insulin-treated diabetes mellitus: its relation to age of onset, sex, and duration of disease. Diabetologia 15:87–93
Soejima K, Landing BH (1986) Osteoporosis in juvenile-onset diabetes mellitus: morphometric and comparative studies. Pediatr Pathol 6:289–299
Einhorn TA, Boskey AL, Gundberg CM, Vigorita VJ, Devlin VJ, Beyer MM (1988) The mineral and mechanical properties of bone in chronic experimental diabetes. J Orthop Res 6:317–323
Shires R, Teitelbaum SL, Bergfeld MA, Fallon MD, Slatopolsky E, Avioli LV (1981) The effect of streptozotocin-induced chronic diabetes mellitus on bone and mineral homeostasis in the rat. J Lab Clin Med 97:231–240
Rosholt MN, Hegarty PVJ (1981) Mineralization of different bones in streptozotocin-diabetic rats: study on the concentration of eight minerals. Am J Clin Nutr 34:1680–1685
Goodman WG, Hori MT (1984) Diminished bone formation in experimental diabetics. Diabetes 33:825–831
Dixit PK, Ekstrom RA (1980) Decreased breaking strength of diabetic rat bone and its improvement by insulin treatment. Calcif Tissue Int 32:195–199
McNair P (1988) Bone mineral metabolism in human type I (insulin-dependent) diabetes mellitus. Danish Med Bull 35:109–121
Griffiths HF (1985) Diabetic osteopathy. Orthopedics 8:401–406
French CE (1943) The interrelation of calcium and fat utilization. J Nutr 25:17–21
Calverley CE, Kennedy C (1949) The effect of fat on calcium and phosphorus metabolism in normal growing rats under a normal dietary region. J Nutr 38:165–175
Kane GG (1949) Dietary fat and calcium wastage in old age. J Gerontol 4:185–192
Beadles JR, Mitchell HH, Hamilton TS (1951) The utilization of dietary calcium by growing albino rats fed diet containing lard or cocoa butter. J Nutr 45:399–405
Givens MH (1917) Studies in calcium and magnesium metabolism. III. The effect of fat and fatty acid derivatives. J Biol Chem 31:441–444
Pepper WF, Slinger SL, Motzok I (1955) Effect of animal fat on the calcium and phosphorus requirement of chicks (abstract) Poult Sci 34:1216
White TW, Grainer RB, Baker FH, Stroud JW (1958) Effect of supplemental fat on digestion and the ruminal calcium requirements of sheep. J Anim Sci 17:797–803
Tadayyon B, Lutwak L (1969) Interrelationship of triglycerides with calcium, magnesium, and phosphorus in the rat. J Nutr 97:246–254
Atteh JO, Leeson S (1983) Effects of dietary fatty acids and calcium levels on performance and mineral metabolism of broiler chickens. Poult Sci 62:2412–2419
Atteh JO, Leeson S (1984) Effects of dietary saturated or unsaturated fatty acids and calcium levels on performance and mineral metabolism of broiler chicks. Poult Sci 63:2252–2260
Boyd OF, Crum CL, Lyman JF (1931) The absorption of calcium soaps and the relation of dietary fat to calcium utilization in the white rat. J Biol Chem 95:29–41
Pelker RR, Friedlaender GE, Markham TC, Panjabi MM, Moen CJ (1984) Effects of freezing and freeze-drying on the biomechanical properties of rat bone. J Orthop Res 1:405–411
Shaw SR, Vailas AC, Grindeland RE, Zernicke RF (1988) Effects of a 1-wk spaceflight on morphological and mechanical properties of growing bone. Am J Physiol 254:R78-R83
Sheehan DC, Hrapchak BB (1980) Theory and practice of histotechnology. C. V. Mosby, St. Louis, pp 89–117
Carter DR (1984) Mechanical loading histories and cortical bone remodeling. Calcif Tissue Int 36:S19-S24
Carter DR (1987) Mechanical loading history and skeletal biology. J Biomech 20:1095–1109
Kream BE, Smith MD, Canalis E, Raisz LG (1985) Characterization of the effect of insulin on collagen synthesis in fetal rat bone. Endocrinology 116:296–302
Littlejohn GO (1985) Insulin and new bone formation in diffuse idiopathic skeletal hyperostosis. Clin Rheumatol 4:294–300
McDoughall EJ (1938) The counteraction by fat of the anticalcifying action of cereals. Biochem J 32:194–202
Knudson A, Floody RJ (1940) Fat as a factor in the healing of rickets with vitamin D. J Nutr 20:317–325
Jones JH (1940) the influence of fat on calcium and phosphorus metabolism. J Nutr 20:367–376
Steggerda FR, Mitchell HH (1951) The calcium balance of adult human subjects on high- and low-fat (butter) diets. J Nutr 45:201–211
Singh L, Gunberg D (1970) Quantitative histology of changes with age in rat bone cortex. J Morphol 133:241–252
Wu K, Schubeck KE, Frost HM, Villanueva A (1970) Haversian bone formation rates determined by a new method in mastodon and in human diabetes mellitus and osteoporosis. Calcif Tissue Res 6:204–219
Frost H (1964) Lamellar bone physiology in diabetes mellitus. An introduction. Henry Ford Hosp Med Bull 12:495–497
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, KC., Zernicke, R.F., James Barnard, R. et al. Effects of a high fat-sucrose diet on cortical bone morphology and biomechanics. Calcif Tissue Int 47, 308–313 (1990). https://doi.org/10.1007/BF02555914
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02555914