Abstract
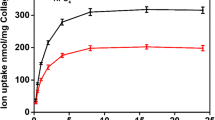
Ultrastructural changes duringin vitro calcification of a collagen-containing matrix derived from beef tendon were correlated with the time of calcification and with the effect of calcification inhibitors. The early stage of calcification is characterized by a molar Ca/P ratio of 1.0–1.2 and by the formation of intrafibrillar electron dense particles. These particles were 50 to 100 Å in diameter and produced no sharp fringes when examined by selected area electron diffraction. During later stages, the electron dense particles appear to coalesce and serve as centers for the growth of hydroxyapatite crystals. At concentration that inhibit hydroxyapatite formation, methylenediphosphonate allows uptake of a small amount of Ca2+ and HPO4 2− with a molar Ca/P of 1.0–1.2 and the formation of the non-crystalline electron dense particles but prevents subsequent growth of hydroxyapatite. Phosphonoacetate depresses Ca2+ and HPO4 2− uptake further and prevents formation of both noncrystalline and crystalline mineral. These findings are consistent with the multistep model of matrix calcification proposed earlier and indicate that the formation of a non-crystalline precursor of hydroxyapatite is induced by the matrix from a soluble phase that is comparable to physiological fluids.
Résumé
Les modifications ultrastructurales au cours de calcificationin vitro d’une matrice de tendon de boeuf, contenant du collagène, sont étudiées en fonction du temps de calcification et de l’efficacité d’inhibiteurs de la calcification. Le stade initial de la calcification est caractérisé par un rapport molaire Ca/P de 1,0–1,2 et par la formation de particules intrafibrillaires, denses aux électrons. Ces particules, de 50 à 100 Å de diamètre, ne donnent pas de raies nettes par diffraction électronique. A des stades plus tardifs, les particules denses aux électrons semblent confluer et servent de centres de croissance aux cristaux d’hydroxyleapatite. A des concentrations qui inhibent la croissance des cristaux d’hydroxyleapatite, le méthylène-diphosphonate permet l’incorporation de faibles quantités de Ca2+ et de HPO4 2−, avec un rapport molaire Ca/P de 1,0–1,2, et la formation de particules non-cristallines, opaques aux électrons. Il empêche, cependant, la croissance ultérieure d’hydroxyleapatite. Le phosphonoacétate diminue encore l’incorporation du Ca2+ et HPO4 2− et empêche la formation du minéral cristallin et non-cristallin. Ces résultats confirment le modèle en plusieurs étapes de la calcification de la matrice et indiquent que la formation d’un précurseur non-cristallin d’hydroxyleapatite est induite par la matrice à partir d’une phase soluble comparable aux liquides physiologiques.
Zusammenfassung
Ultrastrukturelle Veränderungen während der in vitro-Verkalkung einer Collagen enthaltenden Matrix aus Rindersehne wurden mit der Verkalkungszeit und mit der Wirkung von Verkalkungshemmern in Beziehung gebracht. Die frühe Phase der Verkalkung wird charakterisiert durch ein molares Ca/P-Verhältnis von 1,0–1,2 und durch die Bildung von intrafibrillären elektronendichten Partikeln. Diese Partikel hatten einen Durchmesser von 50–100 Å und zeigten keine scharfen Ränder bei der Untersuchung von ausgewählten Zonen mittels Elektronendiffraktion. In späteren Phasen scheinen die elektronendichten Partikel zur verschmelzen und als Zentren für das Wachstum von Hydroxyapatitkristallen zu dienen. Methylendiphosphonat in Konzentrationen, welche die Bildung von Hydroxyapatit hemmen, ermöglicht die Aufnahme einer kleinen Menge von Ca2+ und HPO4 2− mit einem molaren Ca/P von 1,0–1,2 und die Bildung der nicht-kristallinen elektronendichten Partikel, die Aufnahme von Ca2+ und HPO4 2− noch weiter herab und verhindert die Bildung von nichtkristallinem und kristallinem Mineral. Diese Befunde stimmen mit dem früher vorgeschlagenen Multistufenmodell der Matrix-Verkalkung überein und deuten darauf hin, daß die Bildung eines nicht-kristallinen Vorläufers von Hydroxyapatit durch die Matrix aus einer löslichen Phase hervorgerufen wird, welche mit physiologischen Flüssigkeiten vergleichbar ist.
Similar content being viewed by others
References
Bachra, B. N.: Calcificationin vitro of demineralized bone matrix. Calc. Tiss. Res.8, 287–303 (1972).
Fitton-Jackson, S.: The fine structure of developing bone in the embryonic fowl. Proc. roy. Soc. B146, 270–280 (1957).
Fleisch, H., Neuman, W. F.: Mechanisms of calcification: role of collagen, polyphosphate and phosphatase. Amer. J. Physiol.200, 1296–1300 (1961).
Francis, M. D., Russell, R. G. G., Gleisch, H.: The inhibitory effects of diphosphonates on the formation of calcium phosphate crystalsin vitro and on pathological calcificationin vivo. Science165, 1264–1265 (1969).
Frank, R. M., Sognnaes, R. F., Kern, R.: Calcification of dental tissues with special reference to enamel ultrastructure. In: Calcification in biological systems. publication No. 64, R. F. Sognnaes, ed., p. 163–202. Washington: Amer. Assoc. Adv. Sci. 1960.
Glimcher, M. J.: Molecular biology of mineralized tissues with particular reference to bone. Rev. Mod. Physics31, 359–393 (1959).
Glimcher, M. J., Krane, S. M.: The organization and structure of bone and the mechanism of calcification. In: Treatise on collagen, B. S. Gould, ed., vol. 2, p. 68–241. New York: Academic Press 1968.
Jethi, R. K., Inlow, C. W., Wadkins, C. L.: Studies of the mechanism of biological calcification. I.: Kinetic properties of thein vitro calcification of collagen-containing matrix. Calc. Tiss. Res.6, 81–92 (1970).
Jethi, R. K., Wadkins, C. L.: Studies of the mechanism of biological calcification. II: Evidence for a multi-step mechanism of calcification by tendon matrix. Calc. Tiss. Res.7, 277–289 (1971).
Katz, E. P.: The kinetics of mineralizationin vitro. I.: The nucleation properties of 640 Å collagen at 25°C. Biochim. biophys. Acta (Amst.)149, 121–129 (1969).
Luben, R. A., Wadkins, C. L.: Studies of the relationship of proton production and the calcification of tendon matrixin vitro. Biochemistry10, 2183–2189 (1971).
MacGregor, J.: Some observations on the nature of bone mineral. In: Calcified tissue 1965. Proceedings of the Third European Symposium on Calcified Tissues H. Fleisch, H. J. J. Blackwood, and M. Owen, eds. p. 138–142. Berlin-Heidelberg-New York: Springer 1965.
Neuman, W. F., Neuman, M. W.: The chemical dynamics of bone mineral. Chicago: University of Chicago Press 1958.
Nylen, M. V., Scott, D. B., Mosley, V. M.: Mineralization of turkey leg tendon. II.: Collagenmineral relations revealed by electron and x-ray microscopy. In: Calcification in biological systems, R. F. Sognnaes, ed., No 64, p. 129–142. Washington, D. C.: Amer. Assoc. Adv. Sci. publication 1960.
Robinson, R. A., Watson, M. L.: Collagen-crystal relationships in bone as seen in the electron microscope. Anat. Rec.114, 383–396 (1952).
Russell, R. G. G., Muhlbauer, R. C., Biraz, S., Williams, D. A., Fleisch, H.: The influence pyrophosphate, condensed phosphates, phosphonates and other phosphate compounds on the dissolution of hydroxyapatitein vitro and on bone, resorption induced by parathyroid hormone in tissue culture and in thyroparathyroidectomized rats. Calc. Tiss. Res.6, 183–196 (1970).
Schiffman, E., Martin, G. R., Miller, E. J.: Matrices that calcify. In: Biological calcification—cellular and molecular aspects, H. Schraer, ed., p. 27–67. Appleton: Century Crafts 1970.
Sherman, J. K.: Free-thaw-induced latent injury in cells of the renal cortex. Cryobiology6, 98–104 (1969).
Termine, J. D., Posner, A. S.: Infrared analysis of rat bone: age dependency of amorphous and crystalline mineral fractions. Science153, 1523–1525 (1966).
Termine, J. D., Posner, A. S.: Amorphous/crystalline interrelationships in bone mineral. Calc. Tiss. Res.1, 8–23 (1967).
Termine, J. D., Posner, A. S.: Calcium phosphate formationin vitro, I.: Factors affecting initial phase separation. Arch. Biochem.140, 307–317 (1970).
Thomas, W. C., Jr., Tomita, A.: Mineralization of human and bovine tissuein vitro. Amer. J. Path.51, 621–628 (1967).
Wadkins, C. L.: Experimental factors that influence collagen calcificationin vitro. Calc. Tiss. Res.2, 214–228 (1968).
Weber, J. C., Eanes, E. D., Gerdes, R. J.: Electron microscope study of noncrystalline calcium phosphate. Arch. Biochem.120, 723–724 (1967).
West, V. C.: Observations on phase transformation of a precipitated calcium phosphate. Calc. Tiss. Res.7, 212–219 (1971).
Wischnitzer, S.: Introduction to electron microscopy, p. 239–241. New York: Pergamon Press 1970.
Author information
Authors and Affiliations
Additional information
This investigation was supported by United States Public Health Service Grant AM-11528.
Predoctoral fellow of the Department of Biochemistry, University of Arkansas School of Medicine and Trainee of NIH Metabolic Training Program AM-05635. The studies described in this paper constitute a portion of a thesis to be submitted to the Graduate Faculty of the University of Arkansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy.
Rights and permissions
About this article
Cite this article
Luben, R.A., Sherman, J.K. & Wadkins, C.L. Studies of the mechanism of biological calcification. Calc. Tis Res. 11, 39–55 (1973). https://doi.org/10.1007/BF02546594
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02546594