Abstract
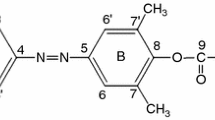
The oxidation of urofuran acid derivatives (1–2) by hypochlorous acid (HOCl) was investigated with the goal to possibly simplify the detection of their metabolites in biological materials. The oxidation products of 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid (1) were obtained as an isomeric mixture and confirmed to exist ascis (3a) andtrans (3b) isomers, based on their13C nuclear magnetic resonance (NMR) spectra. Similarly, the products of 5-H substituted acid 2 obtained by oxidation with HOCl were identified as 4a and 4b by13C and1H NMR which indicated the presence ofcis andtrans hemiacetal hydrogens at C-5 in a ratio of 2.11∶1. The oxidation was found to proceed in a manner different from that of the F-acid, because of the presence of the electron withdrawing COOCH3 group at C-3 which favored the nucleophilic attack on the carbonyl group to affordcis- andtrans-2,5-dihydroxy-2,5-dihydrofurans (3a−b, 4a−b).
Similar content being viewed by others
Abbreviations
- CI-MS:
-
chemical ionization mass spectrometry
- CMPF:
-
3-carboxy-4-methyl-5-propyl-2-furanpropionic acid (1)
- COSY:
-
correlation spectroscopy
- DEPT:
-
distortionless enhancement by polarization transfer
- EI-MS:
-
electron impact ionization mass spectrometry
- FAB-MS:
-
fast atom bombardment mass spectrometry
- GC/MS:
-
gas chromatography/mass spectrometry
- HPLC:
-
highnuclear magnetic resonance
- TLC:
-
thin-layer chromatography
- TMS:
-
tetramethylsilane
References
Spiteller, M., and Spiteller, G. (1979)J. Chromatogr. 164, 253–317.
Liebich, H.M., Pickert, A., and Tetschner, B. (1984)J. Chromatogr. 289, 259–266.
Collier, R., Lindup, W.E., Liebich, H.M., and Spiteller, G. (1986)British J. Clin. Pharmacol. 21, 610–611.
Mabuchi, H., and Nakahashi, H. (1986)Nephron, 44, 277–281.
Hannemann, K., Puchta, V., Simon, E., Ziegler, H., Ziegler, G., and Spiteller, G. (1989)Lipids 24, 296–298.
Sand, D.M., Schlenk, H., Thoma, H., and Spiteller, G. (1983)Biochim. Biophys. Acta 751, 455–461.
Ushijima, Y., and Nakano, M. (1980)Biochem. Biophys. Res. Commun. 93, 1232–1237.
Schödel, R., and Spiteller, G. (1985)Helv. Chim. Acta 68, 1624–1634.
Ishii, K., Okajima, H., Koyamatsu, T., Okada, Y., and Watanabe, H. (1988)Lipids 23, 694–700.
Jandke, J., Schmidt, J., and Spiteller, G. (1988)Liebigs Ann. Chem. 29–34.
Schödel, R., and Spiteller, G. (1987)Liebigs Ann. Chem. 459–462.
Spiteller, M., Spiteller, G., and Hoyer, G.A., (1980)Chem. Ber. 113, 699–709.
Pfordt, J., Thoma, H., and Spiteller, G. (1981)Liebigs Ann. Chem. 2298–2308.
Author information
Authors and Affiliations
About this article
Cite this article
Ohki, T., Maeda, K., Sakakibara, J. et al. Structural analysis of oxidation products of urofuran acid by hypochlorous acid. Lipids 28, 35–41 (1993). https://doi.org/10.1007/BF02536357
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02536357