Abstract
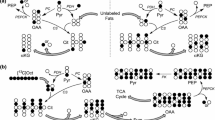
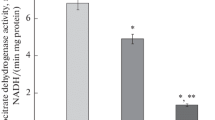
The metabolism of malonaldehyde (MA) was investigated in vivo using male Wistar rats and in vitro using rat liver mitochondria. Twelve hr after intubation with [1,3-14C] MA, 60–70%, 5–15% and 9–17% of administered radioactivity was recovered in expired CO2, feces and urine, respectively. In rats intubated with [1,2-14C] acetate, the corresponding values were 68–82%, 1–2% and 2–3%.14CO2 evolution was initially slower after14C-MA administration than after14C-acetate administration and more radioactivity was excreted in the feces and urine. In vitro experiments using [1,3-14C] MA showed that MA is metabolized primarily in the mitochondria via reactions involving O2 utilization and14CO2 production. The apparent Km and Vmax were 0.5 mM and 9.3 nmol/min/mg protein for O2 uptake, respectively, and 2.0 mM and 2.4 nmol/min/mg protein for14CO2 production. Addition of malonic acid to mitochondrial incubates at concentrations inhibitory to succinate dehydrogenase did not affect MA-induced O2 uptake but enhanced14CO2 production from14C-MA.14C-Acetate appeared to be the major accumulating metabolite in rat liver mitochondrial preparations following a 120-min incubation with14C-MA. A probable biochemical route for MA metabolism involves oxidation of MA by mitochondrial aldehyde dehydrogenase followed by decarboxylation to produce CO2 and acetate.
Similar content being viewed by others
References
Dahle, L.K., Hill, E.G., and Holman, R.T. (1962) Arch. Biochem. Biophys. 98, 253–261.
Pryor, W.A., and Stanley, J.P. (1975) J. Org. Chem. 40, 3615–3617.
Shamberger, R.J., Shamberger, B.A., and Willis, C.E. (1977) J. Nutr. 107, 1404–1409.
Siu, G.M., and Draper, H.H. (1978) J. Food Sci. 43, 1147–1149.
Shin, B.C., Huggins, J.W., and Carraway, K.L. (1972) Lipids 7, 229–233.
Chio, K.S., and Tappel, A.L. (1969) Biochemistry 8, 2827–2832.
Brooks, B.R., and Klamerth, O.L. (1968) Eur. J. Biochem. 5, 178–182.
Reiss, U., Tappel, A.L., and Chio, K.S. (1972) Biochem. Biophys. Res. Commun. 48, 921–926.
Bird, R.P., and Draper, H.H. (1980) J. Toxicol. Environ. Health 6, 811–823.
Mukai, F.H., and Goldstein, B.D. (1976) Science 191, 868–869.
Shamberger, R.J., Corlett, C.L., Beaman, K.D., and Kasten, B.L. (1979) Mutation Res. 66, 349–355.
Bird, R.P., and Draper, H.H. (1979) Fed. Proc. 38, 709.
Shamberger, R.J., Andreone, T.L., and Williss, C.E. (1974) J. Natl. Cancer Inst. 53, 1771–1773.
Bird, R.P., Draper, H.H., and Valli, V.E.O. (1981) Proc. Can. Fed. Biol. Soc., p. 301 (abstract).
Holtkamp, D.E., and Hill, R.M. (1951) Arch. Biochem. Biophys. 34, 216–219.
Recknagel, R.O., and Ghoshal, A.K. (1966) Lab Invest. 15, 132–146.
Placer, Z., Veselkova, A., and Rath, R. (1965) Experientia 21, 19–20.
Horton, A.A., and Packer, L. (1970) J. Gerontol. 25, 199–204.
Protopopova, T.V., and Skoldinov, A.P. (1958) J. Gen. Chem. U.S.S.R. 28, 341–243.
Kwon, T.W., and Watts, B.M. (1963) J. Food Sci. 28, 627–630.
Case, G.L., and Benevenga, N.J. (1976) J. Nutr. 106, 1721–1736.
Chappell, J.B., and Hansford, R.G. (1972) in Subcellular Components (Birnie, G.D., ed.) p. 74, Butterworth & Co. Ltd., Woburn, MA.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J. (1951) J. Biol. Chem. 193, 265–275.
Sinnhuber, R.O., and Yu, T.C. (1958) Food Technol. 12, 9–12.
LaNoue, K., Nicklas, W.J., and Williamson, J.R. (1970) J. Biol. Chem. 245, 102–111.
Horton, A.A., and Barrett, M.C. (1975) Arch. Biochem. Biophys. 167, 426–436.
Chance, B., and Williams, G.R. (1955) J. Biol. Chem. 217, 383–393.
Deitrich, R.A. (1966) Biochem. Pharmacol. 15, 1911–1922.
Menon, G.K.K., Stern, J.R., Kupiecki, F.P., and Coon, M.J. (1960) Biochim. Biophys. Acta 44, 602–604.
Marnett, L.J., and Tuttle, M.A. (1980) Cancer Res. 40, 276–282.
Author information
Authors and Affiliations
About this article
Cite this article
Siu, G.M., Draper, H.H. Metabolism of malonaldehyde in vivo and in vitro. Lipids 17, 349–355 (1982). https://doi.org/10.1007/BF02535193
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02535193