Abstract
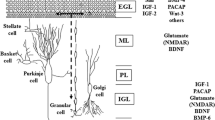
The effect of the depolarizing agents, an elevated potassium concentration (25 mM) or kainic acid (50 μM) on neuronal survival and differentiation was investigated in cultures of dissociated neurons from cerebella of 7-day-old mice. When maintained in the presence of an antimitotic agent such cultures consist primarily of glutamatergic and GABAergic neurons. Cell survival was monitored by measurement of DNA, and differentiation by determining uptake and depolarization coupled release of glutamate (D-aspartate as label) and GABA. The depolarizing agents were added separately or together either from the start of the culture period (7–8 days) or at day 5 in culture. The main findings are that K+ depolarization is important for differentiation of glutamatergic neurons but not for GABAergic neurons. This depolarizing signal is important during the early phase of development in culture. For glutamatergic neurons, kainate may replace K+ as a depolarizing signal whereas in case of the GABAergic neurons, kainate was toxic particularly during the late phase of development. It was further observed that the glutamatergic neurons when maintained in a medium with 5 mM K+ during the first 5 days in culture became sensitive to kainate toxicity when this amino acid was added at day 5. This was not the case when the medium contained 25 mM K+ from the start of the culture period.
Similar content being viewed by others
References
Messer, A. 1977. The maintenance and identification of mouse cerebellar granule cells in monolayer cultures. Brain Res. 130:1–12.
Pearce, B. R., Currie, D. N., Beale, R., and Dutton, G. R. 1981. Potassium-stimulated, calcium-dependent release of [3H]GABA from neuron- and glia-enriched cultures of cells dissociated from rat cerebellum. Brain Res. 206:485–489.
Hertz, L., Juurlink, B. H. J., and Szuchet, S. 1985. Cell cultures. Pages 603–661,in A. Lajtha (ed.) Handbook of Neurochemistry, Vol. 8, Plenum Publ. Corp., New York.
Drejer, J., Larsson, O. M., Kvamme, E., Svenneby, G., Hertz, L., and Schousboe, A. 1985. Ontogenetic development of glutamate metabolizing enzymes in cultured cerebellar granule cells and in cerebellum in vivo. Neurochem. Res. 10:49–62.
Gallo, V., Kingsbury, A., Balázs, R., and Jørgensen, O. S. 1987. The role of depolarization in the survival and differentiation of cerebellar granule cells in culture. J. Neurosci. 7:2203–2213.
Balázs, R., Hack, N., and Jørgensen, O. S. 1988. Stimulation of the N-methyl-D-aspartate receptor has a trophic effect on differentiating cerebellar granule cells. Neurosci. Lett. 87:80–86.
Balázs, R., Jørgensen, O. S., and Hack, N. 1988. N-Methyl-D-aspartate promotes the survival of cerebellar granule cells in culture. Neuroscience 27:437–451.
Balázs, R., Gallo, V., and Kingsbury, A. 1988. Effect of depolarization on the maturation of cerebellar granule cells in culture. Dev. Brain Res. 40:269–279.
Balázs, R., Hack, N., Jørgensen, O. S., and Cotman, C. W. 1989. N-Methyl-D-aspartate promotes the survival of cerebellar granule cells: pharmacological characterization. Neurosci. Lett. 101:241–246.
Peng, L., Juurlink, B. H. J., and Hertz, L. 1991. Difference in transmitter release, morphology, and ischemia-induced cell injury between cerebellar granule cell cultures developing in the presence of and in the absence of a depolarizing potassium concentration. Devl. Brain Res. 63:1–12.
Mogensen, H. S., Hack, N., Balázs, R., and Jørgensen, O. S. 1994. The survival of cultured mouse cerebellar granule cells is not dependent on elevated potassium-ion concentration. Int. J. Devl. Neurosci. 5:451–460.
Drejer, J., and Schousboe, A. 1989. Selection of a pure cerebellar granule cell culture by kainate treatment. Neurochem. Res. 14: 751–754.
Schousboe, A., and Pasantes-Morales, H. 1989. Potassium-stimulated release of3H-taurine from cultured GABAergic and glutamatergic neurons. J. Neurochem. 53:1309–1315.
Kardos, J., Elster, L., Damgaard, I., Krogsgaard-Larsen, P., and Schousboe, A. 1994. Role of GABAB receptors in intracellular Ca2+ homeostasis and possible interaction between GABAA and GABAB receptors in regulation of transmitter release in cerebellar granule neurons. J. Neurosci. Res. 39:646–655.
Palaiologos, G., Hertz, L., and Schousboe, A. 1988. Evidence that aspartate amino transferase activity and ketodicarboxylate carrier function are essential for biosynthesis of transmitter glutamate. J. Neurochem. 51:317–320.
Balázs, R., Hack, N., and Jørgensen, O. S. 1990. Selective stimulations of excitatory amino acid receptor subtypes and the survival of cerebellar granule cells in culture: effect of kainic acid. Neuroscience 37:251–258.
Balázs, R., Hack, N., and Jórgensen, O. S. 1990. Interactive effects involving different classes of excitatory amino-acid receptors and the survival of cerebellar granule cells in culture. Int. J. Dev. Neurosci. 8:347–359.
Graham, M. E., and Burgoyne, R. D. 1994. Activation of metabotropic glutamate receptors by L-AP4 stimulates survival of rat cerebellar granule cells in culture. Eur. J. Pharmacol. 288:115–123.
Drejer, J., Honoré, T., and Schousboe, A. 1987. Excitatory amino acid induced release of3H-GABA from cultured mouse cerebral cortex interneurons. J. Neurosci. 7:2910–2916.
Gram, L., Larsson, O. M., Johnsen, A., and Schousboe, A. 1988. Effects of valproate, vigabatrin and aminooxyacetic acid on release of endogenous and exogenous GABA from cultured neurons. Epilepsy Res. 2:87–95.
Drejer, J., Larsson, O. M., and Schousboe, A. 1983. Characterization of uptake and release processes for D- and L-aspartate in primary cultures of astrocytes and cerebellar granule cells. Neurochem. Res. 8:231–243.
Palaiologos, G., Hertz, L., and Schousboe, A. 1989. Role of aspartate aminotransferase and mitochondrial dicarboxylate transport for release of endogenously and exogenously supplied neurotransmitter in glutamatergic neurons. Neurochem. Res. 14: 359–366.
Belhage, B., Rehder, V., Hansen, G. H., Kater, S. B., and Schousboe, A. 1992.3H-D-Aspartate release from cerebellar granule neurons is differentially regulated by glutamate- and K+-stimulation. J. Neurosci. Res. 33:436–444.
Miller, R. J. 1987. Multiple calcium channels and neuronal function. Science 235:46–52.
Belhage, B., Hansen, G. H., and Schousboe, A. 1993. Depolarization by K+ and glutamate activates different neurotransmitter release mechanisms in GABAergic neurons: Vesicular versus nonvesicular release of GABA. Neuroscience 54:1019–1034.
Westergaard, N., Fosmark, H., and Schousboe, A. 1991. Metabolism and release of glutamate in cerebellar granule cells co-cultured with astrocytes from cerebellum or cerebral cortex. J. Neurochem. 56:59–66.
Westergaard, N., Larsson, O. M., Jensen, B., and Schousboe, A. 1992. Synthesis and release of GABA in cerebral cortical neurons co-cultured with astrocytes from cerebral cortex or cerebellum. Neurochem. Int. 20:567–575.
Schousboe, A., Meier, E., Drejer, J., and Hertz, L. 1989. Preparation of primary cultures of mouse (rat) cerebellar granule cells. Pages 203–206,in A. Shahar, J. De Vellis, A. Vernadakis, and B. Haber (eds.) A dissection and tissue culture manual of the nervous system. Alan R. Liss, New York.
Hertz, L., Juurlink, B. H. J., Fosmark, H., and Schousboe, A. 1982. Astrocytes in primary cultures. Pages 175–186,in S. E. Pfeiffer (ed.) Neuroscience approached through cell culture Vol. 1. CRC Press, Boca Raton, Fl.
Sensenbrenner, M., Maderspach, K., Latzkovits, L., and Jarós, G. G. 1978. Neuronal cells from chick embryo cerebral hemispheres cultivated on polylysine-coated surfaces. Devl. Neurosci. 1:91–101.
Schousboe, A., Svenneby, G., and Hertz, L. 1977. Uptake and metabolism of glutamate in astrocytes cultured from dissociated mouse brain hemispheres. J. Neurochem. 29:999–1005.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurements with the folin phenol reagent. J. Biol. Chem. 193:265–275.
Seiler, N., and Schmidt-Glenewinkel, T. 1975. Regional distribution of putrescine, spermidine and spermine in relation to the distribution of RNA and DNA in the rat nervous system. J. Neurochem. 24:791–795.
Pin, J. P., and Bockaert, J. 1989. Two distinct mechanisms, differentially affected by excitatory amino acids, trigger GABA release from fetal mouse striatal neurons in primary culture. J. Neurosci. 9:6648–656.
Duarte, C. B., Ferreira, I. L., Santos, P. F., Oliveira, C. R., and Carvalho, A. P. 1992. Ca2+-dependent release of [3H]GABA in cultured chick retina cells. Brain Res. 591:27–32.
Frandsen, Aa., and Schousboe, A. 1990. Development of excitatory amino acid induced cytotoxicity in cultured neurons. Int. J. Devl. Neurosci. 8:209–216.
Lasher, R. S., and Zagon, I. S. 1972. The effect of potassium on neuronal differentiation in cultures of dissociated newborn rat cerebellum. Brain Res. 41:482–488.
Thangnipon, W., Kingsbury, A., Webb, M., and Balázs, R. 1983. Observations on rat cerebeller cells in vitro: influence on substratum, potassium concentration and relationship between neurons and astrocytes. Devl. Brain Res. 11:177–189.
Naito, S., and Ueda, T. 1985. Characterization of glutamate uptake into synaptic vesicles. J. Neurochem. 44:99–109.
Hertz, L. 1979. Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Prog. Neurobiol. 13:277–323.
Schousboe, A. 1981. Transport and metabolism of glutamate and GABA in neurons and glial cells. Int. Rev. Neurobiol. 22:1–45.
Kuriyama, K., and Ohkuma, S. 1987. Development of cerebral cortical GABAergic neurons in vitro. Pages 43–56,in, A. Vernadakis, A. Privat, J. M. Lauder, P. S. Timiras, E. Giacobini (eds.) Model Systems of Development and Aging of the Nervous System, Martinus Nijhoff Publishing, Boston.
Trenkner, E., and Sturman, J. A. 1991. The role of taurine in the survival and function of cerebellar cells in cultures of early postnatal cat. Int. J. Devl. Neuroscience 9:77–88.
Maar, T., Moran, J., Schousboe, A., and Pasantes-Morales, H. 1995. Taurine deficiency in dissociated mouse cerebellar cultures affects neuronal migration. Int. J. Devl. Neurosci. 13:491–502.
Author information
Authors and Affiliations
Additional information
Special issue dedicated to Dr. Kinya Kuriyama.
Rights and permissions
About this article
Cite this article
Damgaard, I., Trenkner, E., Sturman, J.A. et al. Effect of K+- and kainate-mediated depolarization on survival and functional maturation of GABAergic and glutamatergic neurons in cultures of dissociated mouse cerebellum. Neurochem Res 21, 267–275 (1996). https://doi.org/10.1007/BF02529144
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02529144