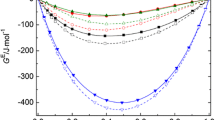
Abstract
The enthalpies of solution ofn-propyl alcohol, ethylene glycol, and glycerol in the H2O—1,4-dioxane and H2O—1,2-dimethoxyethane mixtures were measured in the range of molal concentrations (m alc) of the dissolved alcohol from 0 to 0.1 mol kg−1 at 298.15 K. The enthalpies of solvation of alcohols and the enthalpies of their transfer from water to a mixed aqueous-organic solvent were discussed. The effect of the nature of the studied nonelectrolytes on the characteristics obtained was established. The enthalpy coefficients of binary (h 23) and ternary (h 223) interactions between the molecules of the solute (subscript 3) and the non-aqueous component (subscript 2) of the mixed solvent in the temary solution were calculated. Theh 23 values increase as hydrophobic properties in the alcohol series (glycerol, ethylene glycol, andn-propyl alcohol) increase.
Similar content being viewed by others
References
M. Yu. Panov, V. P. Belousov, and A. G. Morachevskii,Khimiya and termodinamika rastvorov, [Chemistry and Thermodynamics of Solutions], Izd. Leningr. Univ., Leningrad, 1977,4, 213 (in Russian).
S. Nwankwo and I. Wadso,J. Chem. Thermodyn., 1980,12, 1167.
D. J. T. Hill and L. R. White,Austr. J. Chem., 1974.27, 1905.
A. J. Gordon and R. A. Ford,The Chemist's Companion. A Handbook of Practical Data, Techniques, and References, J. Wiley and Sons, New York, 1972.
V. A. Klimova, inOsnovnye mikrometory analiza organicheskikh soedinenii [Basic Methods for Analysis of Organic Compounds], Khimiya, Moscow, 1967, 208 (in Russian).
Yu. A. Karapetyan and V. N. Eichis, inFiziko-Khimicheskie svoistva elektrolitnykh nevodnykh ratvorov [Physiochemical Properties of Aqueous Solutions of Electrolytes], Khimiya, Moscow, 1989, 256 (in Russian).
V. P. Belousov and M. Yu. Panov, inTermodinamika vodnykh rastvorov neelektrolitiv [Thermodynamics of Aqueous Solutions of Electrolytes], Khimiya, Leningard, 1983, 264 (in Russian).
Yu. A. Lebedev and E. A. Miroshnichenko, inTermokhimiya paroobrazovaniya organicheskikh veshchestv [Thermochemistry of Vaporization of Organic Substance], Nauka, Moscow, 1981, 216 (in Russian).
M. M. H. M. Bastos, N. C. M. Dores, R. da Silva, M. A. V. R. da Silva, S. -O. Nilsson, and I. Wadso,Abstrs. 9th IUPAC Conf. on Chemical Thermodynamics, Lisboa (Portugal), 1986, 8.38.
V. P. Belousov and A. G. Morachevskii, inTeploty smesheniya zhidkostei [Hearts of Mixing of Liquids], Khimiya, Leningrad, 1970, 256 (in Russian).
J. R. Goats and R. J. Suillivan,J. Phys. Chem., 1958,62, 188.
E. Tommila and V. Turkki,Suomen Kem., 1967,B40, 207.
D. V. Batov, O. A. Antonova, N. G. Egorova, and V. P. Korolev,Zh. Obshch. Khim., 1996,66, 214 [Russ. J. Gen. Chem., 1996,66 (Engl. Transl.)].
N. G. Manin, O. A. Antonova, and V. P. Korolev,Zh. Obshch. Khim., 1996,66, 1271. [Russ. J. Gen. Chem., 1996,66 (Engl. Transl.)].
Y. Marcus, inIon Solvation, Wiley, New York, 1985, 306.
Yu. Ya. Fialkov,Rastvoritel' kak sredstvo upravleniya khimicheskim protsessom [The Solvent as a Tool for Controlling the Chemical Process], Khimiya, Leningrad, 1990, 240 pp. (in Russian).
Yu. M. Kessler and N. A. Abakumova,Izv. Vuzov, Khim. Khim. Tekhnol. [Bull. High. Educ. Inst., Chem. Chem. Technol.], 1982,25 162 (in Russian).
Yu. M. Kessler, inSovremennye problemy khimii rastvorov [Modern Problems of Solution Chemistry], Nauka, Moscow, 1986, 63 (in Russian).
Yu. M. Kessler and A. L. Zaitsev,Sol'vofobnye effekty. Teoriya, eksperiment, praktika [Solvophobic Effects. Theory, Experiment, Practice], Khimiya, Leningrad, 1989, 312 pp. (in Russian).
W. McMillan and J. Mayer,J. Chem. Phys., 1945,13, 276.
M. V. Kulikov, A. M. Kolker, L. P. Safonova, and Al. G. Krestov,Izv. Akad. Nauk SSSR, Ser. Khim., 1990, 2532 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1990,39, 2289 (Engl. Transl.)].
V. K. Abrosimov,Zh. Khim. Termodin. i Termokhim., [J. Chem. Thermodynam. and Thermochem.], 1992,2, 159 (in Russian).
O. A. Antonova, M. Yu. Kropotova, N. G. Egorova, A. F. Svishchev, D. V. Batov, M. V. Kulikov, and V. P. Korolev,Tez. dokl. VI Mezhdunar. konf. “Problemy sol'vatasii and kompleksoobrazovaniya v rastvorakh” [Abstrs. VIth Int. Conf. “Problems of Solvation and Complexation in Solutions”], Ivanovo, 1995, K-3 (in Russian).
M. V. Kulikov, N. A. Litova, and V. P. Korolev,Tez. dokl. VI Mezhdunar. konf. “Problemy sol'vatatsii and kompleksoobrazovaniya v rastvorakh” [Abstrs. VIth Int. Conf. “Problems of Solvation and Complexation in Solutions”], Ivanovo, 1995, K-34 (in Russian).
A. C. Rouw and G. Somsen,J. Chem. Thermodyn., 1981,13, 67.
D. Hallen, S. -O. Nillson, W. Rothschild, and I. Wadso,J. Chem. Thermodyn., 1986,18, 429.
G. Barone, B. Bove, G. Gastronuovo, and V. Elia,J. Solut. Chem., 1981,10, 803.
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 9, pp. 1592–1599, September, 1997.
Rights and permissions
About this article
Cite this article
Kulikov, M.V., Trutneva, E.Y. Solvation of alcohols with different number of OH groups in mixed solvents H2O—1,4-dioxane and H2O—1,2-dimethoxyethane at 298.15 K. Russ Chem Bull 46, 1518–1525 (1997). https://doi.org/10.1007/BF02502931
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02502931