Abstract
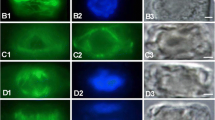
Polarotropism was induced inAdiantum (fern) protonemata grown under polarized red light by turning the electrical vector 45 or 70 degrees. One hour after the light treatment, tropic responses became apparent in many cells as a slight distortion of the apical dome. Changes in the position of the circumferentially-arranged cortical microtubule band (Mt-band) (Murataet al., 1987) and the arrangement of microfibrils around the subapical part of protonemata were investigated in relation to the polarotropic responses. Twenty minutes after turning the electrical vector, preceding the morphological change of cell shape, the Mt-band began to change its orientation from perpendicular to oblique to the initial growing axis. After 30 min, the Mt-band changed its orientation further under 45 degrees polarized light, but under light rotated 70 degrees, it began to disappear. In phototropic responses induced by local irradiation of a side of the subapical part of a protonema with a non-polarized red microbeam, the Mt-band on the irradiated side disappeared or became faint within 20 min, but neither disappearance nor a change of orientation of Mts occurred on the non-irradiated side. One hour after turning the electrical vector 45 degrees, in half of the cells tested, the innermost layer of microfibrils in the subapical part of the protonema changed its orientation from perpendicular to oblique to the growing axis, corresponding to the changes in the orientation of the Mt-band. After 2 hr, those changes were obvious in all cells examined. The same basic results on the orientation of microfibrils were obtained with protonemata cultured for 2 hr under 70 degrees polarized light. The role of the Mt-band in tropic responses is discussed.
Similar content being viewed by others
References
Cyr, R.J. andB.A. Palevitz. 1989. Microtubule-binding proteins from carrot. I. Initial characterization and microtubule bundling. Planta177: 245–260.
Green, P.B. 1980. Organogenesis—A biophysical view. Ann. Rev. Plant Physiol.31: 51–82.
Hartmann, E. 1984. Influence of light on phototropic bending of moss protonemata ofCeratodon purpureus (Hedw.) Brid. J. Hattori Bot. Lab.55: 87–98.
Iino, M., K. Shitanishi, A. Kadota andM. Wada. 1990. Phytochrome-mediated phototropism inAdiantum protonemata—I. Phototropism as a function of the lateral Pfr gradient. Photochem. Photobiol.51: 469–476.
Kadota, A., I. Kohyama andM. Wada. 1989. Polarotropism and photomovement of chloroplasts in the protonemata of the fernPteris andAdiantum: Evidence for the possible lack of dichroic phytochrome inPteris. Plant Cell Physiol.30: 523–531.
—,M. Wada andM. Furuya. 1982. Phytochrome-mediated phototropism and different dichroic orientation of Pr and Pfr in protonemata of the fernAdiantum capillus-veneris L. Photochem. Photobiol.35: 533–536.
—,—and—. 1985. Phytochrome-mediated polarotropism ofAdiantum capillus-veneris L. protonemata as analyzed by microbeam irradiation with polarized light. Planta165: 30–36.
Kakimoto, T. andH. Shibaoka. 1986. Calcium-sensitivity of cortical microtubules in the green algaMougeotia. Plant Cell Physiol.27: 91–101.
Kataoka, H. 1975. Phototropism inVaucheria geminata I. The action spectrum. Plant Cell Physiol.16: 427–437.
Murata, T., A. Kadota, T. Hogetsu andM. Wada. 1987. Circular arrangement of cortical microtubules around the subapical part of a tip-growing fern protonema. Protoplasma141: 135–138.
—, andM. Wada. 1989a. Organization of cortical microtubules and microfibril deposition in response to blue-light induced apical swelling in a tip-growingAdiantum protonema cell. Planta178: 334–341.
——and—. 1989b. Re-organization of microtubules during preprophase band development inAdiantum protonemata. Protoplasma151: 73–80.
Nick, P., P. Bergfeld, E. Schafer andP. Schopfer. 1990. Unilateral reorientation of microtubules at the outer epidermal wall during photo-and gravitropic curvature of maize coleoptiles and sunflower hypocotyls. Planta181: 162–168.
Noguchi, T. andK. Ueda. 1988. Cortical microtubules and cortical microfilaments in the green alga,Micrasterias pinnatifida. Protoplasma143: 188–192.
Olmsted, J.B. 1986. Microtubule-associated proteins. Annu. Rev. Cell Biol.2: 421–457.
Pollard, T.D., S.C. Selden andP. Maupin. 1984. Interaction of actin filaments with microtubules. J. Cell Biol.99: 33s-37s.
Racusen, R.H., H.A. Ketchum andT.J. Cooke. 1988. Modifications of extracellular electric and ionic gradients preceding the transition from tip growth to isodiametric expansion in the apical cell of the fern gametophyte. Plant Physiol.87: 69–77.
Robinson, D.G. andH. Quader. 1982. The microtubule-microfibril syndrome.In C.W. Lloyd, ed. The cytoskeleton in plant growth and development. pp. 109–126. Academic Press, London.
Seagull, R.W. andI.B. Heath. 1979. The effects of tannic acid on the in vivo preservation of microfilaments. Eur. J. Cell Biol.20: 184–188.
Wada, M. andM. Furuya. 1970. Photocontrol of the orientation of cell division inAdiantum. I. Effects of the dark and red periods in the apical cell of gametophytes. Develop. Growth Differ.12: 109–118.
—,A. Kadota andM. Furuya. 1981. Intracellular photoreceptive site for polarotropism in protonema of the fernAdiantum capillus-veneris L. Plant Cell Physiol.22: 1481–1488.
——,—and—. 1983. Intracellular localization and dichroic orientation of phytochrome in plamsa membrane and/or ectoplasm of a centrifuged protonema of fernAdiantum capillus-veneris. Plant Cell Physiol.24: 1441–1447.
Wang, H., A.J. Cutler, M. Saleem andL.C. Fowke. 1989. Microtubules in maize protoplasts derived from cell suspension cultures: effect of calcium and magnesium ions. Eur. J. Cell Biol.49: 80–86.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wada, M., Murata, T. & Shibata, M. Changes in microtubule and microfibril arrangement during polarotropism inAdiantum protonemata. Bot Mag Tokyo 103, 391–401 (1990). https://doi.org/10.1007/BF02491259
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02491259