Abstract
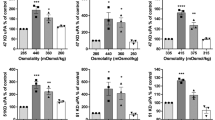
Human glioma cells (T98G and A172 cell lines) were cultured on various extracellular matrix (ECM) components including type 1, IV and V collagens, fibronectin, laminin, and reconstituted basement membrane (Matrigel), and the role of matrix metalloproteinases (MMPs) in their growth and invasion was examined. T98G glioma cells grew well on these ECM components and invaded the reconstituted basement membrane. In contrast, A172 glioma cells showed growth inhibition on collagen types IV and V and Matrigel without invasion of the Matrigel. Gelatin zymography and enzyme immunoassays demonstrated that T98G glioma cells, but not A172 cells, secrete a large amount of matrix metallproteinase-2 (MMP-2, 72 kD gelatinase/type IV collagenase = gelatinase A), and this was confirmed by immunoblotting and immunohistochemistry. Of the two different tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2), T98G cells produced only TIMP-1 during culture on Matrigel, whereas A172 cells secreted both. Although both human recombinant TIMP-1 and TIMP-2 stimulated T98G cell growth slightly on Matrigel, the in vitro invasiveness was significantly reduced by only recombinant TIMP-2. These results suggest that MMP-2 plays an important role in the ECM invasion of T98G human glioma cells in vitro.
Similar content being viewed by others
References
Liotta LA, Steeg PS, Stetler-Stevenson WG: Cancer metastasis and angiogenesis: An imbalance of positive and negative regulation. Cell 64: 327–336, 1991
Brown PD, Bloxidge RE, Stuart NSA, Gatter KC, Carmichael J: Association between expression of activated 72-kilodalton gelatinase and tumor spread in non-small-cell lung carcinoma. J Natl Cancer Inst 85: 574–578, 1993
Davies B, Miles DW, Happerfield LC, Naylor MS, Bobrow LG, Rubens RD, Balkwill FR: Activity of type IV collagenases in benign and malignant breast disease. By J Cancer 67: 1126–1131, 1993
Davies B, Waxman J, Wasan H, Abel P, Williams G, Krausz T, Neal D, Thomas D, Hanby A, Balkwill F: Levels of matrix metalloproteinases in bladder cancer correlate with tumor grade and invasion. Cancer Res 53: 5465–5369, 1993
Naylor MS, Stamp GW, Davies BD, Balkwill FR: Expression and activity of MMPs and their regulators in ovarian cancer. Int J Cancer 58: 50–56, 1994
Stearns ME, Wang M: Type IV collagenase (Mr 72,000) expression in human prostate: Benign and malignant tissue. Cancer Res 53: 878–883, 1993
Apodaca G, Rutka JT, Bouhana K, Berens ME, Giblin JR, Rosenblum ML, McKerrow JH, Banda MJ: Expression of metalloproteinases and metalloproteinase inhibitors by fetal astrocytes and glioma cells. Cancer Res 50: 2322–2329, 1990
Nakagawa T, Kubota T, Kabuto M, Sato K, Kawano H, Hayakawa T, Okada Y: Production of matrix metalloproteinases and tissue inhibitor of metalloproteinases-1 by human brain tumors. J Neurosurg 81: 69–77, 1994
Nakano A, Tani E, Miyazaki K, Furuyama J, Matsumoto T: Expression of matrilysin and stromelysin in human glioma cells. Biochem Biophys Res Commun 192: 999–1003, 1993
Rao JS, Steck PA, Mohanam S, Stetler-Stevenson WG, Liotta LA, Sawaya R: Elevated levels of Mr 92,000 type IV collagenase in human brain tumors. Cancer Res 53: 2208–2211, 1993
Rao JS, Steck PA, Tofilon P, Boyd D, Alim-Osman F, Stetler-Stevenson WG, Liotta LA, Sawaya R: Role of plasminogen activator and of 92-kDa type IV collagenase in glioblastoma invasion using an in vitro matrigel model. J Neurooncol 18: 129–138, 1993
Abe T, Mori T, Kohno K, Seiki M, Hayakawa T, Welgus HG, Hori S, Kuwano M: Expression of 72 kDa type IV collagenase and invasion activity of human glioma cells. Clin Exp Metastasis 12: 296–304, 1994
Rutka JT, Matsuzawa K, Hubbard SL, Furuyama K, Becker LE, Stetler-Stevenson W, Edwards DR, Dirks PB: Expression of TIMP-1, TIMP-2, 72- and 92-kDa type IV collagenase transcripts in human astrocytoma cell lines: correlation with astrocytoma cell invasiveness. Int J Oncol 6: 877–884, 1995
Ohnishi T, Arita N, Hayakawa T, Kawahara K, Kato K, Kakimura A: Purification of motility factor (GMF) from human malignant glioma cells and its biological significance in tumor invasion. Biochem Biophys Res Commun 193: 518–525, 1993
Boone TC, Johnson MJ, DeClerck YA, Langley KE: cDNA cloning and expression of a metalloproteinase inhibitor related to tissue inhibitor of metalloproteinase. Proc Natl Acad Sci USA 87: 2800–2804, 1990
Carmichael DF, Sommer A, Thompson RC, Anderson DC, Smith CG, Welgus HG, Stricklin GP: Primary structure and cDNA cloning of human fibloblast collagenase inhibitor. Proc Natl Acad Sci USA 83: 2407–2411, 1986
Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB: Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res 47: 936–942, 1987
Hibbs MS, Hasty KA, Seyer JM, Kang AH, Mainardi CL: Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem 260: 2493–2500, 1985
Chin JR, Murphy G, Werb Z: Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase: Biosynthesis, isolation, characterization and substrates. J Biol Chem 260: 12367–12376, 1985
Towbin H, Staehelin T, Gordon J: Electrophoretic transfer proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA 76: 4350–4354, 1979
Tanaka K, Iwamoto Y, Ito Y, Ishibashi T, Nakabeppu Y, Sekiguchi M, Sugioka Y: Cyclic AMP-regulated synthesis of the tissue inhibitors of metalloproteinases suppresses the invasive potential of the human fibrosarcoma cell line HT1080. Cancer Res 55: 2927–2935, 1995
Zhang J, Fujimoto N, Iwata K, Sakai T, Okada Y, Hayakawa T: A one-step sandwich immunoassay for human matrix metalloproteinase 1 (interstitial collagenase) using monoclonal antibodies. Clin Chim Acta 219: 1–14, 1993
Fujimoto N, Hosokawa K, Iwata T, Shinya T, Okada Y, Hayakawa T: A one-step sandwich enzyme immunoassay for inactive precursor and complexed forms human matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase, gelatinase B) using monoclonal antibodies. Clin Chim Acta 231: 79–88, 1994
Fujimoto N, Mouri N, Iwata K, Ohuchi E, Okada Y, Hayakawa T: A one-step sandwich enzyme immunoassay for human matrix metalloproteinase 2 (72-kDa gelatinase/type IV collagenase) using monoclonal antibodies. Clin Chim Acta 221: 91–103, 1993
Fujimoto N, Zhang J, Iwata K, Shinya T, Okada Y, Hayakawa T: A one-step sandwich enzyme immunoassay for tissue inhibitor of metalloproteinases-2 using monoclonal antibodies. Clin Chim Acta 220: 31–45, 1993
Kodama S, Iwata K, Iwata H, Yamashita K, Hayakawa T: Rapid one-step sandwich enzyme immunoassay for tissue inhibitor of metalloproteinases. An application for rheumatoid arthritis serum and plasma. J Immunol Methods 127: 103–108, 1990
Obata K, Iwata K, Okada Y, Kohrin Y, Ohuchi E, Yoshida S, Shinmei M, Hayakawa T. A one-step sandwich enzyme immunoassay for human matrix metalloproteinase 3 (stromelysin-1) using monoclonal antibodies. Clin Chim Acta 211: 59–72, 1992
Morré DJ, Morré DM, Mollenhauser HH, Reutter W: Golgi apparatus cisternae of monensin-treated cells accumulate in the cytoplasm of liver slices. Eur J Cell Biol 43: 235–242, 1987
Tartakoff AM: Perturbation of vesicular traffic with carboxylic ionophore monensin. Cell 32: 1026–1028, 1983
Bernstein JJ, Laws ER, Levine KV, Wood LR, Tadvalkar G, Goldberg WJ: C6 glioma-astrocytoma cell and fetal astrocyte migration into artificial basement membrane: A permissive substrate for neural tumors but not fetal astrocytes. Neurosurgery 28: 652–658, 1991
Schlechte W, Brattain M, Boyd D: Invasion of extracellular matrix by cultured colon cancer: Dependence on receptor display. Cancer Commun 2: 173–179, 1990
Goldberg GI, Marmer BL, Grant AZ, Eisen AZ, Wilhelm S, He C: Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci USA 86: 8207–8211, 1989
Boghaert ER, Chan SK, Zimmer C, Grobelny D, Galardy RE, Vanaman TC, Zimmer SG: Inhibition of collagenolytic activity relates to quantitative reduction of invasion in vitro in a c-Ha-ras transfected glial cell line. J Neuro-oncol 21: 141–150, 1994
Hirvonen HE, Salonen R, Sandberg MM, Vuorio E, Västrik I, Kotilainen E, Kalimo H: Differential expression of myc, max and RB1 genes in human gliomas and glioma cell lines. Br J Cancer 69: 16–25, 1994
Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M: A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370: 61–65, 1994
DeClerck YA, Yean TD, Chan D, Shimada H, Langley KE: Inhibition of tumor invasion of smooth muscle cell layers by recombinant human metalloproteinase inhibitor. Cancer Res 52: 2151–2157, 1992
Giese A, Rief MD, Loo MA, Berens ME: Determinants of human astrocytoma migration. Cancer Res 54: 3897–3904, 1994
Paulus W, Torn JC: Basement membrane invasion of glioma cells mediated by integrin receptors. J Neurosurg 80: 515–519, 1994
Merzak A, Koocheckpour S, Pilkington GJ: CD44 mediates human glioma cell adhesion and invasion in vitro. Cancer Res 54: 3988–3992, 1994
Mohanam S, Sawaya R, McCutcheon I, Ali-Osman F, Boyd D, Rao JS: Modulation of in vitro invasion of human glioblastoma cells by urokinase-type plasminogen activator receptor antibody. Cancer Res 53: 4143–4147, 1993
Garbisa S, Scagliotti G, Masiero L, Di Francesco C, Caenazzo C, Onisto M, Micela M, Stetler-Stevenson WG, Liotta LA: Correlation of serum metalloproteinase levels with lung cancer metastasis and response to therapy. Cancer Res 52: 4548–4549, 1992
Baker T, Tickle S, Wasan H, Docherty A, Isenberg D, Waxman J: Serum metalloproteinases and their inhibitors: markers for malignant potential. Br J Cancer 70: 506–512, 1994
Alvarez OA, Carmichael DF, DeClerck YA: Inhibition of collagenolytic activity and metastasis of tumor cells by a recombinant human tissue inhibitor of metalloproteinase. J Natl Cancer Inst 82: 585–595, 1990
Schultz RM, Silberman S, Persky G, Bajkowski AS, Carmichael DF: Inhibition by human recombinant tissue inhibitor of metalloproteinases of human amnion invasion and lung colonization by murine B16-F10 melanoma cells. Cancer Res 48: 5539–5545, 1988
Davies B, Brown PD, East N, Crimmin MJ, Balkwill FR: A synthetic matrix metalloproteinase inhibitor decreases tumor burden and prolongs survival of mice bearing human ovarian carcinoma xenografts. Cancer Res 53: 2087–2091, 1993
Reich R, Thompson EW, Iwamoto Y, Martin GR, Deason JR, Fuller GC, Miskin R: Effects of inhibitors of plasminogen activator, serine proteases and collagenase IV on the invasion of basement membranes by metastasic cells. Cancer Res 48: 3307–3312, 1988
Paganetti PA, Caroni P, Schwab ME: Glioblastoma infiltration into central nervous system tissue in vitro: Involvement of a metalloprotease. J Cell Biol 107: 2281–2291, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakagawa, T., Kubota, T., Kabuto, M. et al. Secretion of matrix metalloproteinase-2 (72 kD gelatinase/type IV collagenase = gelatinase A) by malignant human glioma cell lines: implications for the growth and cellular invasion of the extracellular matrix. J Neuro-Oncol 28, 13–24 (1996). https://doi.org/10.1007/BF00300442
Issue Date:
DOI: https://doi.org/10.1007/BF00300442