Summary
Two computer programs based on simultaneous chemical equilibria were compared for calculation of chemical species in xylem exudates. The first program, CHELATE, was developed to calculate the chemical species in xylem exudates while GEOCHEM was developed to calculate the speciation of natural aquatic systems. The output of the two programs should be similar since they are based on similar calculations. Data input to the programs consisted of concentration data for Ca, Cu, Fe, Mg, Mn, Zn, NH4, PO4, pH and 28 organic ligands reported for xylem exudates from soybean (Glycine max (L.) Merr.) and tomato (Lycopersicon esculentum Mill.) plants grown in nutrient solution17. The organic ligands included amino acids and low molecular weight organic acids (e.g., citric and malic). With the exception of Fe, there were large differences between CHELATE and GEOCHEM in the calculated speciation of nearly all metals in the xylem exudates. In general, there was better agreement between the programs for the speciation of alkaline earth metals than for transition metals. Discrepancies between the two programs were attributed to differences in 1) species considered and 2) stability constants. GEOCHEM considered a greater number of possible complexes. In addition, stability constants for some complexes differed by as much as 10 fold between the two programs. When the data base for GEOCHEM and CHELATE were the same, the output from CHELATE and GEOCHEM was almost identical. Thus, computations performed by the two programs are equally valid, but it is essential that the data base used in chemical models be verified before interpreting the output.
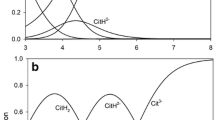
Average concentration data for Al, Au, Ca, Cu, Fe, K, La, Mg, Mn, Na, Rb, Zn, Cl, MoO4, PO4, SO4, HVO4, pH and 18 organic ligands in phloem exudates from Yucca (Yucca flaccida Haw.) were complied from available literature and analyzed by GEOCHEM. Amino acids were the predominant organic ligand analyzed. Calculations revealed that alkali metals existed almost totally as the free ionic species (≥99%) whereas alkaline earth metals were transported as complexes with organic acids (oxalic, malic, and asparagine). Aluminum and Fe were present as hydroxyl species while <1% of micronutrients were transported as the free ion. Major micronutrient species were Cu-glutamine, Mn-asparagine and Zn-alanine. Information on calculated species present in phloem exudates could be useful to guide studies for isolation of metal-ligand complexes in phloem exudates.
Similar content being viewed by others
References
Fellows R J, Egli D B and Leggett J E 1978 A pod leakage technique for phloem translocation studies in soybean (Glycine max (L) Merr). Plant Physiol. 62, 812–814.
Hogfeldt E E 1982 Stability constants of metal-ion complexes. Part A Inorganic ligands. International Union of Pure and Applied Chemists, Pergamon Press, New York.
Griffin R A and Jurinak J J 1983 Estimation of activity coefficients from the electrical conductivity of natural aquatic systems and soil extracts. Soil Sci. 16, 26–30.
Nordstrom D K, Plummer L N, Wigley T M L, Wolery T J, Ball J W, Jenne E A, Bassett R L, Crerar D A, Florence T M, Fritz B, Hoffman M, Holdren G R Jr, Lafon G M, Mattigod J V, Mcduff R E, Morel F, Reddy M M, Sposito G and Thrailkill J 1979 A comparison of computerized chemical models for equilibrium calculations in aqueous systems.In Chemical Modeling in Aqueous Systems. Ed. E A Jenne Am. Chem. Soc., Washington, DC.
Perrin D D 1979 Stability constants of metal-ion complexes. Part B Organic ligands. International Union of Pure and Applied Chemistry, Pergamon Press, New York.
Robson A D and Pitman M G 1983 Interactions between nutrients in higher plants.In Encyclopedia of Plant Physiol New Series 15A Inorganic Plant Nutrition. Eds. A. Lauchi and R L Bieleski, pp 147–180. Springer-Verlag, New York.
Schubert F R 1981 Enzymes of purine biosynthesis and catabolism inGlycine max I Comparison of activities with N2 fixation and composition of xylem exudate during nodule development. Plant Physiol. 68, 1115–1122.
Sillen L G and Martell A E 1964 Stability constants of metal-ion complexes. The Chemical Society Burlington House, London.
Sillen L G and Martell A E 1971 Stability constants of metal-ion complexes. Supplement No 1 The chemical Society, Burlington House, London.
Sposito G and Mattigod S V 1979 GEOCHEM: A computer program for calculating chemical equilibria in soil solutions and other natural water systems. Kearney Foundation of Soil Sci., Univ. of California, Riverside.
Streeter J G 1979 Allantoin and allantoic acid in tissues and stem exudate from field-grown soybean plants. Plant Physiol. 63, 478–480.
Streeter J G 1982 Synthesis and accumulation of nitrite in soybean nodules supplied with nitrate. Plant Physiol. 69, 1429–1434.
Tammes P M L and Van Die J 1964 Studies on phloem exudation fromYucca Flaccida Haw I Some observations on the phenomenon of bleeding and the composition of the exudate. Acta Bot. Neerl 13, 76–83.
Tiffin L O 1972 Translocation of micronutrients in plants.In Micronutrients in Agriculture. Eds. J J Mortvedt, Giordano P M and Lindsay W L, Am. Soc. Agron., Madison, pp 199–229.
Van Die J and Tammes P M L 1975 Phloem exudation from monocotyledonous axes.In Encyclopedia of Plant Physiol. New Series. I. Phloem Transport. Eds. M H Zimmerman and J A Milburn. pp 200–268. Springer-Verlag, Berlin-Heidelburg-New York.
White M C 1979 Metal chelation in xylem fluid. PhD Thesis, University of Maryland.
White M C, Baker F D, Chaney R L and Decker A M 1981 Metal complexation in xylem fluid. II. Theoretical equilibrium model and computational computer program. Plant Physiol. 67, 301–310.
White M C, Chaney R L and Decker A M 1981 Metal complexation in xylem fluid. II. Electrophoresis evidence. Plant Physiol. 67, 311–315.
White M C, Decker A M and Chaney R L 1981 Metal complexation in xylem fluid. I. Chemical composition of tomato and soybean stem exudate. Plant Physiol. 67, 292–300.
Wolterbeek B and Van Die J 1980 The contents of some hitherto not reported trace elements in phloem exudate fromYucca Flaccida Haw, determined by means on non-destructive Neutron activation analysis. Acta Bot. Neerl. 29, 307–309.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mullins, G.L., Sommers, L.E. & Housley, T.L. Metal speciation in xylem and phloem exudates. Plant Soil 96, 377–391 (1986). https://doi.org/10.1007/BF02375142
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02375142