Abstract
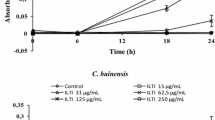
The antifungal spectrum and fungicidal mechanism of an N-terminal peptide of bovine lactoferrin (lactoferricin B), an antimicrobial peptide produced by gastric pepsin digestion of bovine lactoferrin, were investigated. The susceptibility of pathogenic yeasts and dermatophytes to the peptide varied in a species-dependent and strain-dependent manner. Dematiaceous fungi and dimorphic fungi were susceptible to the peptide (range of MIC values: 0.63 to 10 μg/mL). In the case of nonpigmented hyphomycetes and zygomycetes, most strains exhibited resistance to the peptide (MIC:>80 μg/ mL). This peptide killedCandida albicans dose dependently without inducing a change in cell wall stability against osmotic stress. The peptide at 10 μg/ml immediately induced the release of K+ fromC. albicans cells and pH increases in cell suspensions. These pharmacological activities were more potent than those for miconazole nitrate, a well-known antifungal agent that interferes with membrane synthesis and function. These in vitro findings suggest that the lactoferrin oligopeptide has potent membrane disrupting activity against this yeast and suggests that in vivo LF-B studies would be useful to further understand host defenses and to develop improved therapeutic agents against yeast infections.
Similar content being viewed by others
References
Brock JH. Lactoferrin in human milk: its role in iron absorption and protection against enteric infection in newborn infants. Arch Dis Child 1980;55:417–421.
Reiter B. The biological significance of lactoferrin. Int J Tissue React 1983;5:87–96.
Sánchez L, Miguel C, Brock JH. Biological role of lactoferrin. Arch Dis Child 1992;67:657–661.
Soukka T, Tenovuo J, Lenander-Lumikari M. Fungicidal effect of human lactoferrin againstCandida albicans. FEMS Microbiol Lett 1992;69:223–228.
Palma C, Cassone A, Serbousek D, Pearson CA, Djeu JY. Lactoferrin release and interleukin-1, interleukin-6, and tumor necrosis factor production by human polymorphonuclear cells stimulated by various lipopolysaccharides: relationship to growth inhibition ofCandida albicans. Infect Immun 1992;60:4604–4611.
Tomita M, Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K. Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J Dairy Sci 1991;74:4137–4142.
Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta 1992;121:130–136.
Bellamy W, Takase M, Wakabayashi H, Kawase K, Tomita M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J Appl Bacteriol 1992;73:472–479.
Jones EM, Smart A, Bloomberg G, Burgess L, Millar MR. Lactoferricin, a new antimicrobial peptide. A Appl Bacteriol 1994;77:208–214.
Bellamy W, Wakabayashi H, Takase M, Kawase K, Shimamura S, Tomita M. Kining ofCandida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med Microbiol Immunol 1993;182:97–105.
Bellamy W, Yamauchi K, Wakabayashi H, Takase M, Takakura N, Shimamura S, Tomita M. Antifungal properties of lactoferricin B, a peptide derived from the N-terminal region of bovine lactoferrin. Lett Appl Microbiol 1994;18:230–233.
Itoyama T, Aoki Y, Hiratani T, Uchida K, Yamaguchi H. In vitro antifungal activity of omoconazole nitrate, a new imidazole antimycotic. J Antibiot (Tokyo) 1993;46:773–780.
Yamaguchi H, Hiratani T, Iwata K, Yamamoto Y. Studies of the mechanism of antifungal action of aculeacin A. J Antibiot (Tokyo) 1982;35:210–219.
Hiratani T, Yamaguchi H. Studies on the mode of antifungal action of bifonazole. Chemotherapy (Tokyo) 1984;32:829–841.
Cope JE. Mode of action of miconazole onCandida albicans: effects on growth, viability and K+ release. J Gen Microbiol 1980;19:245–251.
Tomita M, Takase M, Bellamy W, Shimamura S. A review: The active peptide of lactoferrin. Acta Paediatr Jpn 1994;36:585–591.
Author information
Authors and Affiliations
About this article
Cite this article
Wakabayashi, H., Hiratani, T., Uchida, K. et al. Antifungal spectrum and fungicidal mechanism of an N-terminal peptide of bovine lactoferrin. J Infect Chemother 1, 185–189 (1996). https://doi.org/10.1007/BF02350646
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02350646