Abstract
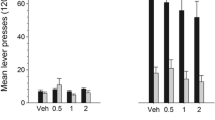
It has been suggested that the dopamine D1 receptor may play an important role in reward. The present study was undertaken to investigate the roles of dopamine D1 and D2 receptor subtypes in responding for conditioned reward. This was done by examining the effects of the D1 antagonist SCH 23390 and the D2 antagonists pimozide and metoclopramide on amphetamine-produced enhancement of responding for conditioned reward. The procedure consisted of three distinct phases. During the pre-exposure phase the rats were exposed to an operant chamber containing two levers. One lever produced a lights-off stimulus (3 s) and the other a tone stimulus (3 s). This was followed by four conditioning sessions during which the levers were removed and the rats were exposed to pairings of the lights-off stimulus with food. This phase was followed by two test sessions during which the levers were present and the number of responses made on each was calculated as a ratio of the number of responses made during the pre-exposure phase. A group receiving the vehicle during the test sessions showed a greater ratio of responding for the light-off stimulus than the tone stimulus, indicating that the lights-off stimulus had become a conditioned reward. Amphetamine (0.1, 1.0, 2.0 and 5.0 mg/kg, IP, 5 min prior to test) specifically enhanced responding on the lever producing conditioned reward. SCH 23390 (5.0 and 10.0 µg/kg, SC, 2 h before test) and pimozide (0.1 and 0.2 mg/kg, IP, 4 h before test) dose-dependently shifted the peak in the amphetamine dose-response function to the right, indicating an attenuation of conditioned reward. Metoclopramide (1.0, 5.0 and 7.5 mg/kg, IP, 1 h before test) reduced the amphetamine-produced enhancement of responding for conditioned reward but failed to shift the amphetamine dose-response function. These results provide evidence that both D1 and D2 receptor subtypes are essential in responding for conditioned reward.
Similar content being viewed by others
References
Beninger RJ (1983) The role of dopamine in locomotor activity and learning. Brain Res Rev 6:173–196
Beninger RJ (1989a) Methods for determining the effects of drugs on learning. In: Boulton AA, Baker GB, Greenshaw AJ (eds) Neuromethods, Volume 13: Psychopharmacology. Humana Press, Clifton, NJ, pp 623–685
Beninger RJ (1989b) Dissociating the effects of altered dopaminergic function on performance and learning. Brain Res Bull 23:365–371
Beninger RJ (1991) Receptor subtype-specific dopamine agonists and antagonists and conditioned behaviour. In: Willner P, Scheel-Kruger J (eds) The Mesolimbic Dopamine System: From Motivation to Action. Wiley, Chichester, pp 273–299
Beninger RJ (1992) D-1 receptor involvement in reward-related learning. J Psychopharmacol 6[1]:34–42
Beninger RJ, Phillips AG (1980) The effects of pimozide on the establishment of conditioned reinforcement. Psychopharmacology 68:147–153
Beninger RJ, Ranaldi R (1992) The effects of amphetamine, apomorphine, SKF 38393, quinpirole and bromocriptine on responding for conditioned reward in rats. Behav Pharmacol 3:155–163
Beninger RJ, Hanson DR, Phillips AG (1980) The effects of pipradrol on the acquisition of responding with conditioned reinforcement: a role for sensory preconditioning. Psychopharmacology 69:235–242
Beninger RJ, Hanson DR, Phillips AG (1981) The acquisition of responding with conditioned reinforcement: effects of cocaine, (+)-amphetamine and pipradrol. Br J Pharmacol 74:149–154
Beninger RJ, Cheng M, Hahn BL, Hoffman DC, Mazurski EJ, Morency MA, Ramm P, Stewart RJ (1987) Effects of extinction, pimozide, SCH 23390, and metoclopramide on food-rewarded operant responding of rats. Psychopharmacology 92:343–349
Beninger RJ, Hoffman DC, Mazurski EJ (1989) Receptor subtype-specific dopaminergic agents and conditioned behavior. Neurosci Biobehav Rev 13:113–122
Bindra D (1974) A motivational view of learning, performance, and behavior modification. Psychol Rev 81:199–213
Bischoff S, Heinrich M, Sonntag JM, Krauss J (1986) The D-1 dopamine receptor antagonist SCH 23390 also interacts potently with brain serotonin (5-HT2) receptors. Eur J Pharmacol 129:367–370
Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC (1986) Increased dopamine metabolism in the nucleus accumbens and striatum following consumption of a nutritive meal but not a palatable non-nutritive saccharin solution. Pharmacol Biochem Behav 25:1095–1100
Cador M, Taylor JR, Robbins TW (1991) Potentiation of the effects of reward-related stimuli by dopaminergic-dependent mechanisms in the nucleus accumbens. Psychopharmacology 104:377–385
Chu B, Kelley AE (1992) Potentiation of reward-related responding by psychostimulant infusion into nucleus accumbens: role of dopamine receptor subtypes. Psychobiology 20:153–162
Cohen SL, Branch MN (1991) Food-paired stimuli as conditioned reinforcers: effects ofd-amphetamine. J Exp Anal Behav 56:277–288
Corrigall WA, Coen KM (1991) Cocaine self-administration is increased by both D1 and D2 dopamine antagonists. Pharmacol Biochem Behav 39:799–802
Costall B, Naylor RJ (1976) A comparison of the abilities of typical neuroleptic agents and of thioridazine, clozapine, sulpiride and metoclopramide to antagonise the hyperactivity induced by dopamine applied intracerebrally to areas of the extrapyramidal and mesolimbic systems. Eur J Pharmacol 40:9–19
Elliott PNC, Jenner P, Huizing G, Marsden CD, Miller R (1977) Substituted benzamides as cerebral dopamine antagonists in rodents. Neuropharmacology 16:333–342
Everitt BJ, Robbins TW (1992) Amygdala-ventral striatal interactions and reward-related processes. In: Aggleton JP (ed) The amygdala. Wiley-Liss, New York, pp 401–429
Fenton HM, Liebman JM (1982) Self-stimulation response decrement patterns differentiate clonidine, baclofen and dopamine antagonists from drugs causing performance deficit. Pharmacol Biochem Behav 17:1207–1212
Files FJ, Branch MN, Clody D (1989) Effects of methylphenidate on responding under extinction in the presence and absence of conditioned reinforcement. Behav Pharmacol 1:113–121
Goetsch VL, Isaac W (1983) The effects ofd-amphetamine on visual sensitivity in the rat. Eur J Pharmacol 87:465–468
Harrington RA, Hamilton CW, Brogden RN, Linkewich JA, Romankiewicz JA, Heel RC (1983) Metoclopramide: an updated review of its pharmacological properties and clinical use. Drugs 25:451–494
Hernandez L, Hoebel BG (1988) Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci 42:1705–1712
Hicks PE, Schoemaker H, Langer SZ (1984) 5-HT receptor antagonist properties of SCH 23390 in vascular smooth muscle and brain. Eur J Pharmacol 105:339–343
Hill RT (1970) Facilitation of conditioned reinforcement as a mechanism of psychomotor stimulation. In: Costa E, Garatinni S (eds) Amphetamines and related compounds. Raven Press, New York, pp 781–795
Hiroi N, White NM (1991) The amphetamine conditioned place preference: differential involvement of dopamine receptor subtypes and two dopaminergic terminal areas. Brain Res 552:141–152
Hoffman DC, Beninger RJ (1985) The effects of pimozide on the establishment of conditioned reinforcement as a function of the amount of conditioning. Psychopharmacology 87:454–460
Hyttel J (1983) SCH 23390 — the first selective dopamine D1 antagonist. Eur J Pharmacol 91:153–154
Iorio LC (1983) SCH 23390, a benzazepine with atypical effects on dopaminergic systems. Pharmacologist 23:136
Isaac W (1971) A study of the relationship between the visual system and the effects ofd-amphetamine. Physiol Behav 6:157–159
Jenner P, Clow A, Reavill C, Theodorou A, Marsden CD (1978) A behavioural and biochemical comparison of dopamine receptor blockade produced by haloperidol with that produced by substituted benzamide drugs. Life Sci 23:545–550
Kelley AE, Delfs JM (1991a) Dopamine and conditioned reinforcement: II. Contrasting effects of amphetamine microinjection into the nucleus accumbens with peptide microinjection into the ventral tegmental area. Psychopharmacology 103:197–203
Kelley AE, Delfs JM (1991b) Dopamine and conditioned reinforcement: I. Differential effects of amphetamine microinjections into striatal subregions. Psychopharmacology 103:187–196
Kelley AE, Delfs JM, Chu B (1990) Neurotoxicity induced by the D-1 agonist SKF 38393 following microinjection into rat brain. Brain Res 532:342–346
Keppel G (1982) Design and Analysis: A Researcher's Handbook. 2nd edition. Prentice-Hall, Englewood Cliffs, New Jersey
Kleven MS, Woolverton WL (1990) Effects of continuous infusions of SCH 23390 on cocaine- or food-maintained behavior in rhesus monkeys. Behav Pharmacol 1:365–373
Knott PD, Clayton KN (1966) Durable secondary reinforcement using brain stimulation as the primary reinforcer. J Comp Physiol Psychol 61:151–153
Koob GF, Simon H, Herman JP, Le Moal M (1984) Neuroleptic-like disruption of the conditioned avoidance response requires destruction of both the mesolimbic and nigrostriatal dopamine systems. Brain Res 303:319–329
Koob GF, Le HT, Creese I (1987) The D1 dopamine receptor antagonist SCH 23390 increases cocaine self-administration in the rat. Neurosci Lett 79:315–320
Kurumiya S, Nakajima S (1988) Dopamine D1 receptors in the nucleus accumbens: involvement in the reinforcing effect of tegmental stimulation. Brain Res 448:1–6
Le Moal M, Simon H (1991) Mesocorticolimbic dopaminergic netwok: functional and regulatory roles. Physiol Rev 71:155–234
Mackintosh NJ (1974) The psychology of animal learning. Academic Press, London
Maidment NT, Marsden CD (1987) Acute administration of clozapine, thioridazine and metoclopramide increases extracellular DOPAC and decreases extracellular 5-HIAA, measured in the nucleus accumbens and striatum of the rat using in vivo voltammetry. Neuropharmacology 26:187–193
Mazurski EJ, Beninger RJ (1986) The effects of (+)-amphetamine and apomorphine on responding for a conditioned reinforcer. Psychopharmacology 90:239–243
Miller R, Wickens JR, Beninger RJ (1990) Dopamine D-1 and D-2 receptors in relation to reward and performance: a case for the D-1 receptor as a primary site of therapeutic action of neuroleptic drugs. Prog Neurobiol 34:143–183
Milner PM (1977) Theories of reinforcement, drive and motivation. In: Iversen LL, Iversen SD, Snyder SH (eds) Handbook of psychopharmacology. Principles of behavioral pharmacology. Plenum Press, New York, pp 181–200
Mogenson GJ, Jones DL, Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14:69–97
Nakahara D, Ozaki N, Kapoor V, Nagatsu T (1989) The effect of uptake inhibition on dopamine release from the nucleus accumbens of rats during self- or forced stimulation of the medial forebrain bundle: a microdialysis study. Neurosci Lett 104:136–40
Nakajima S (1986) Suppression of operant responding in the rat by dopamine D1 receptor blockade with SCH 23390. Physiol Psychol 14:111–114
Nakajima S, Baker JD (1989) Effects of D2 dopamine receptor blockade with raclopride on intracranial self-stimulation and food-reinforced operant behaviour. Psychopharmacology 98:330–333
Nakajima S, McKenzie GM (1986) Reduction of the rewarding effect of brain stimulation by a blockade of dopamine D1 receptor with SCH 23390. Pharmacol Biochem Behav 24:919–923
Nakajima S, O'Regan NB (1991) The effects of dopaminergic agonists and antagonists on the frequency-response function for hypothalamic self-stimulation in the rat. Pharmacol Biochem Behav 39:465–468
Phillips AG, Pfauss JG, Blaha CD (1991) Dopamine and motivated behavior: insights provided by in vivo analyses. In: Willner P, Scheel-Kruger J (eds) The mesolimbic dopamine system: from motivation to action. Wiley, Chichester, pp 199–224
Radhakishun FS, van Ree JM, Westerink BH (1988) Scheduled eating increases dopamine release in the nucleus accumbens of food-deprived rats as assessed with on-line brain dialysis. Neurosci Lett 85:351–356
Robbins TW (1975) The potentiation of conditioned reinforcement by psychomotor stimulant drugs: a test of Hill's hypothesis. Psychopharmacologia 45:103–114
Robbins TW (1976) Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs. Nature 264:57–59
Robbins TW (1978) The acquisition of responding with conditioned reinforcement: effects of pipradrol, methylphenidate,d-amphetamine, and nomifensine. Psychopharmacology 58:79–87
Robbins TW, Koob GF (1978) Pipradrol enhances reinforcing properties of stimuli paired with brain stimulation. Pharmacol Biochem Behav 8:219–222
Robbins TW, Watson BA, Gaskin M, Ennis C (1983) Contrasting interactions of pipradrol,d-amphetamine, cocaine, cocaine analogues, apomorphine and other drugs with conditioned reinforcement. Psychopharmacology 80:113–119
Roberts DCS, Vickers G (1984) Atypical neuroleptics increase self administration of cocaine: an evaluation of a behavioural screen for antipsychotic activity. Psychopharmacology 82:135–139
Rotrosen J, Stanley M, Lautin A, Wazer D, Gershon S (1981) Discrimination of functionally heterogeneous receptor subpopulations: antipsychotic and antidopaminergic properties of metoclopramide. Psychopharmacol Bull 17:110–113
Scheel-Kruger J (1971) comparative studies of various amphetamine analogues demonstrating different interactions with the metabolism of the catecholamines in the brain. Eur J Pharmacol 14:47–59
Seeman P (1981) Brain dopamine receptors. Pharmacol Rev 32:229–313
Self DW, Stein L (1992) The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Res 582:349–352
Skinner, BF (1938) The behavior of organisms. Appleton Century Crofts, New York
Stein L (1958) Secondary reinforcement established with subcortical stimulation. Science 127:466–467
Taylor JR, Robbins TW (1984) Enhanced behavioural control by conditioned reinforcers following microinjections ofd-amphetamine into the nucleus accumbens. Psychopharmacology 84:405–412
Taylor JR, Robbins TW (1986) 6-hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbensd-amphetamine. Psychopharmacology 90:390–397
Ungerstedt U (1979) Central dopamine mechanisms and unconditioned behaviour. In: Horn AS, Korf J, Westerink BHC (eds) The neurobiology of dopamine. Academic Press, London, pp 577–596
Westerink BHC (1979) The effects of drugs on dopamine biosynthesis and metabolism in the brain. In: Horn AS, Korf J, Westerink BHC (eds) The neurobiology of dopamine. Academic Press, London, pp 255–291
White NM, Milner PM (1992) The psychobiology of reinforcers. Annu Rev Psychol 43:443–471
White NM, Packard MG, Hiroi N (1991) Place conditioning with dopamine D1 and D2 agonists injected peripherally or into nucleus accumbens. Psychopharmacology 103:271–276
Winer BJ (1971) Statistical Principles in Experimental Design. 2nd edition. McGraw Hill, New York
Winocour G, Chawla K, Sampson D, Sophokleous S, Muscat R (1988) Tests of functional equivalence between pimozide pretreatment, extinction and free feeding. Psychopharmacology 95:423–426
Wise RA, Rompre P-P (1989) Brain dopamine and reward. Annu Rev Psychol 40:191–225
Woolverton WL, Goldberg LI, Ginos JZ (1984) Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther 230:678–683
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ranaldi, R., Beninger, R.J. Dopamine D1 and D2 antagonists attenuate amphetamine-produced enhancement of responding for conditioned reward in rats. Psychopharmacology 113, 110–118 (1993). https://doi.org/10.1007/BF02244342
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02244342