Abstract
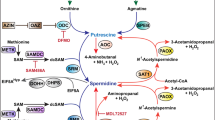
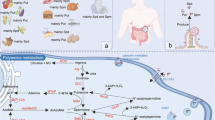
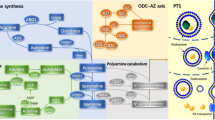
We investigated the polyamine levels [putrescine (Put), spermidine (Spd), and spermine (Spm)] and their metabolism by simultaneously considering the ornithine decarboxylase (ODC) and diamine oxidase (DAO) activities in human colorectal cancer and in normal surrounding tissue. Single and total polyamine levels were significantly higher in the neoplastic tissue than in the surrounding mucosa from the same patients. Furthermore, the ODC activity was significantly higher and the DAO activity significantly lower in the neoplastic tissue than in the surrounding mucosa. Polyamine levels and enzymatic activities did not correlate with the clinical and histologic characteristics of patients. In normal tissue samples, no correlation was found between single and total polyamine levels and enzymatic activities (both DAO and ODC). On the contrary, in colorectal neoplastic samples, significant and positive correlations were found between the levels of total polyamines, Spd, and Spm and the ODC activity. In the same specimens, DAO activity was related to Spd levels and the Spd/Spm ratio, but, in those cases, the correlation was negative. Thus, our findings suggest that, during the neoplastic growth of the colorectal mucosa, the balance between polyamine degradation and biosynthesis is disengaged from the control exerted by the two enzymes.
Similar content being viewed by others
References
Kingsnorth AN, Lumsden AB, Wallace HM. Polyamines in colorectal cancer. Br J Surg 1984;71:791–4.
Tabor CW, Tabor H. Polyamines. Annu Rev Biochem 1984;53:749–90.
Saydjari R, Townsend C Jr, Barranco SC, Thompson JC. Polyamines in gastrointestinal cancer. Dig Dis Sci 1989;34:1629–36.
Williams-Ashman HG, Cannelakis ZN. Polyamines in mammalian biology and medicine. Perspect Biol Med 1979;2:421–53.
Pegg AE, McCann PP. Polyamine metabolism and function. Am J Physiol 1982;243:c212-c221.
Porter CW, Herrera-Ornelas L, Pera P, Petrelli NF, Mittelman A. Polyamine biosynthetic activity in normal and neoplastic human colorectal tissues. Cancer 1987;60:1275–81.
Beaven MA, DeJang W. Presence of histaminase and ornithyne decarboxylase activities in rat thymus. Biochem Pharmacol 1973;22:257–65.
McCormack SA, Johnson LR. Role of polyamines in gastrointestinal mucosal growth. Am J Physiol 1991;260:G795-G806.
Luk GD, Bayless JM, Baylin SB. Diamine oxidase (histaminase): a circulating marker for rat intestinal mucosa maturation and integrity. J Clin Invest 1980;66:66–70.
Mennigen R, Kusche J, Krakamp B,et al. Large bowel tumors and diamine oxidase (DAO) activity in patients: a new approach for risk group identification. Agents Actions 1988;23:351–3.
Mennigen R, Leisten L, Kusche J. Inhibition of polyamine metabolism and mucosal hyperproliferation as risk factors of large bowel cancer in rats. Eur Surg Res 1987;19(Suppl 1):99.
Lointer P, Pezet D, Roch AM, Quash G, Chipponi J. Erreurs du metabolism des polyamines dans les polypes et les adenocarcinomes colorectaux d'origine humaine. Gastroenterol Clin Biol 1989;13:946–7.
Russo F, Linsalata M, Messa C,et al. Polyamines and estrogen receptor concentrations in human colorectal carcinomas. Ital J Gastroenterol 1992;24:8–12.
Di Leo A, Linsalata M, Cavallini A, Messa C, Russo F. Sex steroid hormone receptors, epidermal growth factor receptor, and polyamines in human colorectal cancer. Dis Colon Rectum 1992;35:305–9.
Ginty DD, Seidel ER. Polyamine-dependent growth and calmodulin-regulated induction of ornithine decarboxylase. Am J Physiol 1989;256:6342–8.
Biondi PA, Secchi C, Negri A, Tedeschi G, Ronchi S. Improved high-performance liquid Chromatographic determination of diamine oxidase activity. J Chromatogr 1989;491:209–14.
Lowry OH, Rosebrough NJ, Farr AL, Randall RS. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–7.
La Muraglia GM, Lacaine F, Malt RA. High ornithine decarboxylase activity and polyamine levels in human colorectal neoplasia. Ann Surg 1986;204:89–93.
Holm I, Persson L, Stjenborg L, Thorsson L, Heby O. Feedback control of ornithine decarboxylase expression by polyamines. Biochem J 1989;258:343–50.
Persson L, Holm I, Heby O. Regulation of ornithine decarboxylase mRNA translation by polyamines. J Biol Chem 1988;263:3528–33.
Author information
Authors and Affiliations
About this article
Cite this article
Linsalata, M., Russo, F., Cavallini, A. et al. Polyamines, diamine oxidase, and ornithine decarboxylase activity in colorectal cancer and in normal surrounding mucosa. Dis Colon Rectum 36, 662–667 (1993). https://doi.org/10.1007/BF02238593
Issue Date:
DOI: https://doi.org/10.1007/BF02238593