Abstract
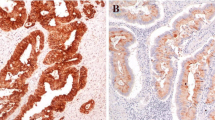
PURPOSE: Recently, endoscopic mucosal resection has been performed commonly for colorectal tumors. However, incomplete endoscopic mucosal resection produces a residual tumor that grows rapidly. The aim of this study was to clarify the characteristics of the residual tumor using the nude mouse model. METHODS: Human colon cancer cells (colo201 or colo320DM) were implanted subcutaneous into nude mice. We then removed more than one-half of the tumor with an electrocautery snare or a surgical knife, and compared the tumor growth rate with that of control tumors. Before and after resection, we examined the Ki-67 labeling index of the tumors with an immunohistochemical assay and mRNA expression for epidermal growth factor receptor, vascular endothelial growth factor, and transforming growth factor alpha. RESULTS: Residual tumors showed a higher growth rate in tumor volume than control tumors using both methods (electrocautery snare and surgical knife). Colo201 groups showed a higher total volume change per day than colo320DM groups after resection. Furthermore, these tumors also showed a higher Ki-67 labeling index, and a stronger epidermal growth factor receptor and transforming growth factor alpha mRNA expression than primary and control tumors in the colo201 implanted groups. There was no significant difference in vascular endothelial growth factor mRNA expression between groups implanted with colo201 or colo320DM. CONCLUSION: Our results suggest that residual tumors caused by incomplete endoscopic mucosal resection may have a higher growth potential than the tumors before resection.
Similar content being viewed by others
References
Chantereau MJ, Faivre J, Bourtron MC,et al. Epidemiology, management, and prognosis of malignant large bowel polyps within a defined population. Gut 1992;33:259–63.
Bedogni G, Bertoni G, Ricci E,et al. Colonoscopic excision of large and giant colorectal polyps: technical implications and results over eight years. Dis Colon Rectum 1986;29:831–5.
Walsh RM, Ackroyd FW, Shellito PC. Endoscopic resection of large sessile colorectal polyps. Gastrointest Endosc 1992;38:303–9.
Binmoeller KF, Bohnacker S, Seifert H, Thonke F, Valdeyar H, Soehndra N. Endoscopic snare excision of “giant” colorectal polyps. Gastrointest Endosc 1996;43:183–8.
Okamoto H, Sasaki T, Tsubomizu Y, Satake Y, Fujita R. Overlooked colonic neoplastic polyps after endoscopic polypectomy [in Japanese with English abstract]. Gastroenterol Endosc 1989;31:1241–6.
Christie JP. Colonoscopic excision of large sessile polyps. Am J Gastroenterol 1977;67:430–8.
Strealovsky VP. Results of endoscopic removal of villous tumors of the colon. Endoscopy 1983;15:49–52.
Nivatvongs S, Snover DC, Fang DT. Piecemeal snare excision of large sessile colon and rectal polyps: is it adequate? Gastrointest Endosc 1984;30:18–20.
Kitajima N, Yamashita Y, Fujimi T,et al. A colonic polyp showing interesting endoscopic and pathologic features after endoscopic polypectomy [in Japanese with English abstract]. Endosc Dig 1994;6:1493–8.
Tanaka S, Haruma K, Tanimoto T,et al. Ki-67 and transforming growth factor alpha (TGF-α) expression in colorectal recurrent tumors after endoscopic resection. Advances in Gastroenterological Carcinogenesis I. Bologna: Monduzzi Editore, 1996:1079–83.
Nishiyama M, Takagami S, Kirihara Y,et al. The indications of chemosensitivity tests against various anti-cancer agents. Surg Today 1988;18:647–52.
Nishiyama M, Saeki S, Aogi K, Hirabayashi N, Toge T. Relevance of DT-diaphorase activity to mitomycin C (MMC) efficacy on human cancer cells: differences in in vitro and in vivo systems. Int J Cancer 1993;53:1013–6.
Ovejena AA, Houchens DP, Baker AD. Chemotherapy of human tumor xenografts in genetically athymic mice. Ann Clin Lab Sci 1978;8:50–6.
Britten CD, Hilsenbeck SG, Eckhardt SG,et al. Enhanced antitumor activity of 6-hydroxymethylacylfulvene in combination with Irinotecan and 5-Fluorouracil in the HT29 human colon tumor xenograft model. Cancer Res 1999;59:1049–53.
Teixeira CR, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G. Proliferating cell nuclear antigen expression at the invasive tumor margin predicts malignant potential of colorectal carcinomas. Cancer 1994;73:575–9.
Tanaka S, Haruma K, Tatsuta S,et al. Proliferating cell nuclear antigen expression correlates with the metastatic potential of submucosal invasive colorectal carcinoma. Oncology 1995;52:134–9.
Aoki R, Tanaka S, Haruma K,et al. Muc-1 expression as a predictor of the curative endoscopic treatment of submucosally invasive colorectal carcinoma. Dis Colon Rectum 1998;41:1262–71.
Reed JA, Manahan LJ, Park CS, Brigati DJ. Complete one-hour immunocytochemistry based on capillary action. Biotechniques 1992;13:434–43.
Kitadai Y, Ellis LM, Takahashi Y,et al. Multiparametricin situ messenger RNA hybridization analysis to detect metastasis-related genes in surgical specimens of human colon carcinomas. Clin Cancer Res 1995;1:1095–102.
Kitadai Y, Bucana CD, Ellis LM, Anzai H, Tahara E, Fidler IJ. In situ mRNA hybridization technique for analysis of metastasis-related genes in human colon carcinoma cells. Am J Pathol 1995;147:1238–47.
Radinsky R, Bucana CD, Ellis LM,et al. A rapid colorimetric in situ messenger RNA hybridization technique for analysis of epidermal growth factor receptor in paraffin-embedded surgical specimens of human colon carcinomas. Cancer Res 1993;53:937–43.
Bucana CD, Radinsky R, Dong Z, Sanchez R, Brigati DJ, Fidler IJ. A rapid colorimetric in situ mRNA hybridization technique using hyperbiotinylated oligonucleotide probes for analysis of mdr1 in mouse colon carcinoma cells. J Histochem Cytochem 1993;41:499–506.
Yamamoto S, Yasui W, Kitadai Y,et al. Expression of vascular endothelial growth factor in human gastric carcinomas. Pathol Int 1998;48:499–506.
Yamamoto G, Tari A, Sumii K, Sumii M, Haruma K, Kajiyama G. Famotidine, a histamine-2-receptor antagonist, inhibits the increase in rat gastric H+/K+-ATPase mRNA induced by intravenous infusion of gastrin 17, and histamine. Dig Dis Sci 1995;40:2064–9.
Kitadai Y, Ellis LM, Tucker SL,et al. Multiparametric in situ mRNA hybridization analysis to predict disease recurrence in patients with colon carcinoma. Am J Pathol 1996;149:1541–51.
Bradly SJ, Garfinkle G, Walker E, Salem R, Chen LB, Steele G. Increased expression of the epidermal growth factor receptor on human colon carcinoma cells. Arch Surg 1986;121:1242–7.
Radinsky R, Risin S, Fan D,et al. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res 1995;1:19–31.
Barnard JA, Beauchamp RD, Russel WE, Dubois RN, Coffey RJ. Epidermal growth factor related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology 1995;108:564–80.
Tanaka S, Imanishi K, Haruma K,et al. Immunoreactive transforming growth factor-α and epidermal growth factor in colorectal adenomas and carcinomas. Oncology 1992;49:381–5.
Tanaka S, Imanishi K, Yoshihara M,et al. Immunoreactive transforming growth factor alpha is commonly present in colorectal neoplasia. Am J Pathol 1991;139:123–9.
Yasui W, Sumiyoshi H, Hata J,et al. Expression of epidermal growth factor receptor in human gastric and colonic carcinomas. Cancer Res 1988;48:137–41.
Sporn MB, Todaro GJ. Autocrine secretion and malignant transformation of cells. N Engl J Med 1980;303:878–80.
Ito M, Yoshida K, Kyo E,et al. Expression of several growth factors and their receptor genes in human colon carcinomas. Virchows Arch B Cell Pathol 1990;59:173–8.
Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G. H. A. Clowes Memorial Award Lecture. Cancer Res 1986;46:467–73.
Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990;82:4–6.
Kim KJ, Li B, Winer J,et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature 1993;362:841–4.
Takahashi Y, Mai M, Wilson MR, Kitadai Y, Bucana CD, Ellis LM. Site-dependent expression of vascular endothelial growth factor, angiogenesis and proliferation in human gastric carcinoma. Int J Oncol 1996;8:701–5.
Berse B, Brown L, Van De Water L, Dvorak H, Senger DR. Vascular permeability gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell 1992;3:211–20.
De Vries C, Escobido JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992;255:989–91.
Ferrara N, Houck KA, Jakeman LB, Winer J, Leung WD. The vascular endothelial growth factor family of polypeptides. J Cell Biochem 1991;47:211–8.
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246:1306–9.
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 1984;133:1710–5.
Key G, Becker MH, Barton B,et al. New Ki-67-equivalent murine monoclonal antibodies (MIB1-3) generated against bacterially expressed parts of the Ki-67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab Invest 1993;68:629–36.
Gerdes J, Becker MH, Key G, Cattoretti G. Immunohistological detection of tumor growth fraction (Ki-67 antigen) in formalin-fixed and routinely processed tissues. J Pathol 1992;168:85–6.
Tarnawski A, Stachura J, Durbin T, Sarfeh IJ, Gergely H. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology 1992;102:695–8.
Polk WH Jr, Dempsey PJ, Russell WE,et al. Increased production of transforming growth factor-α following acute gastric injury. Gastroenterology 1992;102:1467–74.
Luck MS, Bass P. Effect of epidermal growth factor on experimental colitis in rats. J Pharmacol Exp Ther 1993;264:984–90.
Kajikawa K, Yasui W, Sumiyoshi H,et al. Expression of epidermal growth factor in human tissues. Virchows Arch [A] 1994;418:27–32.
Koretz K, Schlag P, Moller P. Expression of epidermal growth factor receptor in normal colorectal mucosa, adenoma and carcinoma. Virchows Arch [A] 1990;416:343–9.
Borlinghaus P, Wieser S, Lamerz R. Epidermal growth factor, transforming growth factor-α and epidermal growth factor receptor content in normal and carcinomatous gastric and colonic tissue. Clin Invest 1993;71:903–7.
Suzuki Y, Ohtani H, Mizoi T,et al. Cell adhesion molecule expression by vascular endothelial cells as an immune/inflammatory reaction in human colon carcinoma. Jpn J Cancer Res 1995;86:585–93.
Yoshida K, Tsujino T, Yasui W,et al. Induction of growth factor-receptor and metalloproteinase genes by epidermal growth factor and/or transforming growth factor-α in human gastric carcinoma cell line MKN-28. Jpn J Cancer Res 1990;81:793–8.
Author information
Authors and Affiliations
About this article
Cite this article
Kunihiro, M., Tanaka, S., Haruma, K. et al. Electrocautery snare resection stimulates cellular proliferation of residual colorectal tumor. Dis Colon Rectum 43, 1107–1115 (2000). https://doi.org/10.1007/BF02236558
Issue Date:
DOI: https://doi.org/10.1007/BF02236558