Abstract
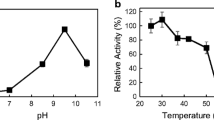
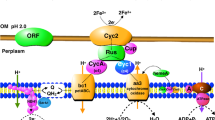
Many N2-fixing organisms can turn off nitrogenase activity in the presence of NH4 + and turn it on again when the NH4 + is exhausted. One of the most interesting systems for accomplishing this is by covalent modification of one subunit of dinitrogenase reductase by dinitrogenase reductase ADP-ribosyltransferase (DRAT). The system can be reactivated when NH4 + is exhausted, by dinitrogenase reductase activating glycohydrolase (DRAG) which removes the inactivating group. It is fascinating that some species of the genusAzospirillum possess the DRAT and DRAG systems (A. lipoferum andA. brasilense), whereasA. amazonense in the same genus lacks DRAT and DRAG.A. amazonense responds to NH4 + but does not exhibit modification of dinitrogenase reductase characteristic of the action of DRAT. However, it has been possible to clone DRAT and DRAG and to introduce them intoA. amazonense, whereupon they become functional in this organism. The DRAT and DRAG system does not appear to function inAcetobacter diazotrophicus, an organism isolated from sugar cane, that fixes N2 at a pH as low as 3.0.A. diazotrophicus does show a rather sluggish response to NH4 +. A level of about 10 μM NH4 + is required to ‘switch off’ the system. The response to NH4 + is influenced by the dissolved oxygen concentration (DOC) as has been reported forAzospirillum sp. A DOC in equilibrium with 0.1 to 0.2 kPa O2 seems optimal for the response inA. diazotrophicus.
Similar content being viewed by others
References
Becking J H 1963 Fixation of molecular nitrogen by an aerobicVibrio orSpirillum. Antonic van Leeuwenhoeck 29, 326.
Beijerinck M W 1925 Über einSpirillum welches freien Stickstoff binden kann? Zenthl. Bakt. Parasitkde. 63, 353–359.
Boddey R M and Döbereiner J 1988 Nitrogen fixation associated with grasses and cereals: Recent results and perspectives for future research. Plant and Soil 108, 53–65.
Burris R H and Wilson P W 1946 Ammonia as an intermediate in nitrogen fixation byAzotobacter. J. Bacteriol. 52, 505–512.
Cejudo F J, de la Torre A and Paneque A 1984 Short-term ammonium inhibition of nitrogen fixation inAzotobacter. Biochem. Biophys. Res. Commun. 123, 431–437.
Döbereiner J 1989 Isolation and identification of root-associated diazotrophs.In Nitrogen Fixation with Non-Legumes. Eds. F ASkinner et al. pp 103–108. Kluwer Academic Publishers, Dordrecht, The Netherlands.
Döbereiner J and Day J M 1976 Associative symbioses in tropical grasses: Characterization of microorganisms and dinitrogen-fixing sites.In Proc. 1st Internat. Symp. on Nitrogen Fixation. Eds. W ENewton and C JNyman. pp 518–538. Washington State University Press, Pullman, WA.
Fu H and Burris R H 1989 Ammonium inhibition of nitrogenase activity inHerbaspirillum seropedicae. J. Bacteriol. 171, 3168–3175.
Fu H, Burris R H and Roberts G P 1990 Reversible ADP-ribosylation is demonstrated to be a regulatory mechanism in prokaryotes by heterologous expression. Proc. Natl. Acad. Sci. USA 87, 1720–1724.
Fu H, Fitzmaurice W P, Roberts G P and Burris R H 1990 Cloning and expression ofdraTG genes fromAzospirillum lipoferum. Gene 86, 95–98.
Fu H, Hartmann A, Lowery R G, Fitzmaurice W P, Roberts G P and Burris R H 1989 Posttranslational regulatory system for nitrogenase activity inAzospirillum spp. J. Bacteriol. 171, 4679–4685.
Fu H-A, Wirt H J, Burris R H and Roberts G P 1989 Functional expression of aRhodospirillum rubrum gene encoding dinitrogenase reductase ADP-ribosyltransferase in enteric bacteria. Gene 85, 153–160.
Gest H and Kamen M D 1949 Photoproduction of molecular hydrogen byRhodospirillum rubrum. Science 109, 558–559.
Hartmann A and Burris R H 1987 Regulation of nitrogenase activity by oxygen inAzospirillum brasilense andAzospirillum lipoferum. J. Bacteriol. 169, 944–948.
Hartmann A, Fu H and Burris R H 1986 Regulation of nitrogenase activity by ammonium chloride inAzospirillum spp. J. Bacteriol. 165, 864–870.
Hartmann A, Fu H and Burris R H 1988 Influence of amino acids on nitrogen fixation ability and growth ofAzospirillum spp. Appl. Environ. Microbiol. 54, 87–93.
Jouanncau Y, Meyer C and Vignais P M 1988 Regulation of nitrogenase activity inRhodobacter capsulatus: ADP ribosylation of the Fe protein.In Nitrogen Fixation: Hundred Years After. Eds. HBothe, F JdeBruijn and W ENewton. p. 173. Gustav Fischer, Stuttgart.
Kamen M D and Gest H 1949 Evidence for a nitrogenase system in the photosynthetic bacteriumRhodospirillum rubrum. Science 109, 560.
Ludden P W 1980 Nitrogen fixation by photosynthetic bacteria: Properties and regulation of the enzyme system fromRhodospirillum rubrum.In Nitrogen Fixation. Eds. W ENewton and W HOrme-Johnson. pp 139–156. University Press, Baltimore, MD.
Ludden P W and Burris R H 1976 Activating factor for the iron protein of nitrogenase fromRhodospirillum rubrum. Science 194, 424–426.
Ludden P W and Burris R H 1978 Purification and properties of nitrogenase fromRhodospirillum rubrum, and evidence for phosphate, ribose and an adenine-like unit covalently bound to the iron protein. Biochem. J. 175, 251–259.
Ludden P W and Burris R H 1979 Removal of an adenine-like molecule during activation of dinitrogenase reductase fromRhodospirillum rubrum. Proc. Natl. Acad. Sci. USA 76, 6201–6205.
Ludden P W, Hageman R V, Orme-Johnson W H and Burris R H 1982 Properties and activities of ‘inactive’ Fe protein fromRhodospirillum rubrum. Biochim. Biophys. Acta 700, 213–216.
Ludden P W, Okon Y and Burris R H 1978 The nitrogenase system ofSpirillum lipoferum. Biochem. J. 173, 1001–1003.
Ludden P W and Roberts G P 1989 Regulation of nitrogenase activity by reversible ADP ribosylation.In Current Topics in Cellular Regulation 30, 23–56. Academic Press, New York.
Nordlund S and Eriksson U 1979 Nitrogenase fromRhodospirillum rubrum. Relation between ‘switch-off’ effect and the membrane component. Hydrogen production and acetylene reduction with different nitrogenase component ratios. Biochim. Biophys. Acta 547, 429–437.
Nordlund S, Eriksson U and Baltscheffsky H 1977 Necessity of a membrane component for nitrogenase activity inRhodospirillum rubrum. Biochim. Biophys. Acta 462, 187–195.
Okon Y, Albrecht S L and Burris R H 1976 Factors affecting growth and nitrogen fixation ofSpirillum lipoferum. J. Bacteriol. 127, 1248–1254.
Okon Y, Houchins J P, Albrecht S L and Burris R H 1977 Growth ofSpirillum lipoferum at constant partial pressures of oxygen, and the properties of its nitrogenase in cell-free extracts. J. Gen. Microbiol. 98, 87–93.
Pope M R, Murrell S A and Ludden P W 1985a Purification and properties of the heat-released nucleotide-modifying group from the inactive iron protein of nitrogenase fromRhodospirillum rubrum. Biochemistry 24, 2374–2380.
Pope M R, Murrell S A and Ludden P W 1985b Covalent modification of the iron protein of nitrogenase fromRhodospirillum rubrum by adenosine diphosphoribosylation of a specific arginine residue. Proc. Natl. Acad. Sci. USA 82, 3173–3177.
Pope M R, Saari L L and Ludden P W 1986 N-glycohydrolysis of adenosine diphosphoribosyl arginine linkages by dinitrogenase reductase activating glycohydrolase (activating enzyme) fromRhodospirillum rubrum. J. Biol. Chem. 261, 10104–10111.
Schneider K C, Bradbeer C, Singh R N, Wang L C, Wilson P W and Burris R H 1960 Nitrogen fixation by cell-free preparations from microorganisms. Proc. Natl. Acad. Sci. USA 46, 726–733.
Song S-D, Hartmann A and Burris R H 1985 Purification and properties of the nitrogenase ofAzospirillum amazonense. J. Bacteriol. 164, 1271–1277.
Sweet W J and Burris R H 1981 Inhibition of nitrogenase activity by NH4 + in Rhodospirillum rubrum. J. Bacteriol. 145, 824–831.
Vignais P M, Willison J C, Allibert P, Ahombo G and Jouanneau Y 1987 Regulation of nitrogen fixation in photosynthetic bacteria.In Inorganic Nitrogen Metabolism. Eds. OUllrich et al. pp. 154–159. Springer-Verlag, Berlin.
Wilson P W, Hull J F and Burris R H 1943 Competition between free and combined nitrogen in nutrition ofAzotobacter. Proc. Natl. Acad. Sci. USA 29, 289–294.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burris, R.H., Hartmann, A., Zhang, Y. et al. Control of nitrogenase inAzospirillum sp.. Plant Soil 137, 127–134 (1991). https://doi.org/10.1007/BF02187443
Issue Date:
DOI: https://doi.org/10.1007/BF02187443