Abstract
The functional properties of a purified homogeneous spinach PS II-core complex with high oxygen evolution capacity (Haag et al. 1990a) were investigated in detail by measuring thermoluminescence and oscillation patterns of flash induced oxygen evolution and fluorescence quantum yield changes. The following results were obtained:
-
a)
Depending on the illumination conditions the PS II-core complexes exhibit several thermoluminescence bands corresponding to the A band, Q band and Zv band in PS II membrane fragments. The lifetime of the Q band (Tmax=10°C) was determined to be 8s at T=10°C. No B band corresponding to S2QB − or S3QB − recombination could be detected.
-
b)
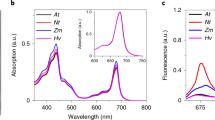
The flash induced transient fluorescence quantum yield changes exhibit a multiphasi relaxation kinetics shich reflect the reoxidation of Q −A . In control samples without exogenous acceptors this process is markedly slower than in PS II membrane fragments. The reaction becomes significantly retarded by addition of 10 μM DCMU. After dark incubation in the presence of K3[Fe(CN)6
-
c)
Excitation of dark-adapted samples with a train of short saturating flashes gives rise to a typical pattern dominated by a high O2 yield due to the third flash and a highly damped period four oscillation. The decay of redox states S2 and S3 are dominated by short life times of 4.3 s and 1.5 s, respectively, at 20°C.
The results of the present study reveal that in purified homogeneous PS II-core complexes with high oxygen evolution isolated from higher plants by β-dodecylmaltoside solubilization the thermodynamic properties and the kinetic parameters of the redox groups leading to electron transfer from water to QA are well preserved. The most obvious phenomenon is a severe modification of the QB binding site. The implications of this finding are discussed.
Similar content being viewed by others
Abbreviations
- β-DM:
-
β-dodecyl-maltoside
- PS II:
-
Photosystem II
- RC:
-
reaction center
- QA, QB :
-
Primary and secondary quinone acceptors
- Ph-p-BQ:
-
phenylparabenzoquinone
- Chl:
-
chlorophyll
- TL:
-
thermoluminescence
- LHC II:
-
Photosystem II light-harvesting antenna complex
- MES:
-
4-morpholineethanesulfonic acid
- HEPES:
-
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- DCMU:
-
3-(3,4-dichlorophenyl)-1-1-dimethylurea
- DCBQ:
-
dichlorobenzoquinone
- Si :
-
sequential oxidation states of the water oxidizing enzyme in the model of B. Kok (i=0...4)
- YZ :
-
tyrosine 161 of D1 protein, which is the secondary donor of Photosystem II
References
Barber J, Chapman DJ and Telfer A (1987) Characterization of a PS II reaction centre isolated from the chloroplasts ofPisum sativum. FEBS Lett 220: 67–73
Bernarding J, Wiese N, Eckert H-J, Eichler H-J, Haag E and Renger G (1991) Picosecond measurements of small UV-absorption changes of oxygen evolving Photosystem II core particles. In: Harris GD (ed) Proc. of the Int. Congr. on Lasers, pp 162–167. San Diego
Berthold DA, Babcock GT and Yocum CA (1981) A highly resolved oxygen-evolving Photosystem II preparation from spinach thylakoid membranes. FEBS Lett 134: 231–234
Bowlby NR, Ghanotakis DF, Yocum CF, Petersen J and Babcock GT, (1989) In: Stevens SE and Bryant DA (eds) Light Energy Transduction in Photosynthesis: Higher Plants & Bacterial Models, 215–226. American Society of Plant Physiologists, Rockville, Maryland USA
Brettel K and Witt HT (1983) Reaction kinetics of the photooxidized chlorophylla 11 in chloroplasts measured in the nanosecond range at 837 nm under repetitive flash excitation. Photobiochem Photobiophys 6: 253–260
Chen R (1969) On the calculation of activation energies and frequency factors from glow curves. J Appl Phys 40: 570–585
Debus RJ, Feher G and Okamura MY (1985) LM complex of reaction centers fromRhodopseudomonas sphaeroides R-26: Characterization and reconstitution with the H subunit. Biochemistry 24: 2488–2500
Dekker JP, Boekema EJ, Witt HT and Rögner M (1988) Refined purification and further characterization of oxygen-evolving and Tris-treated Photosystem II particles from the thermophilic cyanobacteriumsynechococcus sp. Biochim Biophys Acta 936: 307–318
DeVault D and Govindjee (1989) Photosynthetic glow peaks and their relationship with the free energy changes. Photosynth Res 24: 175–181
Diner BA and Petrouleas V (1988) Q400 the non-heme iron of Photosystem II iron-quinone complex. A spectroscopic probe of quinone and inhibitor binding to the reaction center. Biochim Biophys Acta 895: 107–125
Eckert H-J, Wiese N, Bernarding J, Eichler H-J. and Renger G (1988) Analysis of the electron transfer from Pheo− to QA in PS II membrane fragments from spinach by time resolved 325 nm absorption changes in the picosecond domain. FEBS Lett 240: 153–158
Eckert H-J, Liu B-N, Geiken B, Eichler H-J and Renger G (1992) Photoinhibition of electron transfer through PS II as studied by time resolved flash absorption spectroscopy. Photosynthetica, in press
Franzen LG, Styring S, Etienne AL, Hansson Ö and Vernotte C (1986) Spectroscopic and functional characterization of a highly oxygen-evolving Photosystem II reaction center complex from spinach. Photobiochem Photobiophys 13: 15–28
Ghanotakis DF, Waggoner CM, Bowlby NR, Demetriou DM, Babcock GT and Yocum CF (1987) Comparative structural and catalytic properties of oxygen-evolving Photosystem II preparations. Photosynth Res 14: 191–199
Gleiter HM (1988) Impulsfluorimetrische Detektion von Photosynthesereaktionen. Diploma Thesis (in German), Technical University Berlin
Gleiter HM, Haag E and Renger G (1990) Temperature dependence of the PS II reaction pattern detected by flash induced fluorescence changes. In: Baltscheffsky M (ed) Current Research in Photosynthesis, Proc. of the VIIIth Int. Congr. on Photosynthesis, Stockholm, Vol I, pp 479–482. Kluwer Academic Publishers, Dordrecht
Haag E, Irrgang KD, Boekema EJ and Renger G (1990a) Functional and structural analysis of PS II core complexes from spinach with high oxygen evolution capacity. Eur J Biochem 189: 47–53
Haag E, Boekema EJ, Irrgang KD and Renger G (1990b) Functional and structural characterization of a highly oxygen evolving PS II-core complex from spinach. In: Baltscheffsky M (ed) Current Research in Photosynthesis, Proc. of the VIIIth Int. Congr. on Photosynthesis, Stockholm, Vol I, pp 375–378. Kluwer Academic Publishers, Dordrecht
Haag E, Gleiter HM and Renger G (1992) Effects of photoinhibition on the PS II acceptor side including the endogenous high spin Fe2+ in thylakoids, PS II membrane fragments and PS II-core complexes. Photosynth Res 131: 113–126
Hansson Ö and Wydrzynski T (1990) Current perceptions of Photosystem II. Photosynth Res 23: 131–162
Ichikawa T, Inoue Y and Shibata K (1975) Characteristics of thermoluminescence bands of intact leaves and isolated chloroplasts in relation to the watersplitting activity in photosynthesis. Biochim Biophys Acta 408: 228–239
Ikeuchi M, Yuasa M and Inoue Y (1985) Simple and discrete isolation of an O2-evolving PS II reaction center complex retaining Mn and the extrinsic 33 kDa protein. FEBS Lett 185: 316–322
Ikeuchi M and Inoue Y (1986) Characterization of O2 evolution by a wheat Photosystem II reaction center complex isolated by a simplified method: Disjunction of secondary acceptor quinone and enhanced Ca2+ demand. Arch Biochem Biophys 247: 97–107
Irrgang KD, Boekema EJ, Vater J and Renger G (1988) Structural determination of the Photosystem II core complex from spinach. Eur J Biochem 178: 209–217
Joliot A and Joliot P (1964) Étude cinétique de la réaction photochimique libérant l'oxygène au cours de la photosynthèse. CR Acad Sci Paris 258: 4622–4625
Joliot P (1972) Modulated light source use with the oxygen electrode. Methods Enzymol 24: 123–134
Joliot P and Kok B (1975) Oxygen evolution in photosynthesis. In: Govindjee (ed) Bioenergetics of Photosynthesis, pp 387–412. Academic Press, New York
Koike H, Siderer Y, Ono T-A, Inoue Y (1986) Assignment of thermoluminescence A band to S3QA − charge recombination: Sequential stabilization of S3 and QA − by a two-step illumination at different temperatures. Biochim Biophys Acta 850: 80–89
Koike H and Inoue Y (1987) Temperature dependence of the S-state transition in a thermophilic cyanobacterium measured by thermoluminescence. In: Biggins J (ed) Progress in Photosynthesis Research, Proc. of the VIIth Int. Congr. on Photosynthesis, Providence, Vol. I, pp 645–648. Rhode Island
Lübbers K and Junge W (1990) Is the proton release due to water oxidation directly coupled to events in the manganese center. In: Baltscheffsky M (ed) Current Research in Photosynthesis, Proc. of the VIIIth Int. Congr. on Photosynthesis, Stockholm, Vol I, pp 877–880. Kluwer Academic Publishers, Dordrecht
Messinger J (1988) Die Wirkung exogener Redoxsubstanzen auf das Oszillationsmuster der O2-Bildung in PS II Membranfragmenten. Diploma Thesis (in German), Technical University, Berlin
Messinger J and Renger G (1990) Reactivity of hydrazine with Photosystem II strongly depends on the redox state of the water-oxidizing system. FEBS Lett 227: 141–146
Michel H and Deisenhofer J (1988) Relevance of the photosynthetic reaction center from purple bacteria to the structure of Photosystem II. Biochemistry 27: 1–7
Nanba O and Satoh K (1987) Isolation of a Photosystem II reaction center consisting of D1 and D2 polypeptides and cytochromeb 559. Proc Natl Acad Sci USA 84: 109–112
Renger G (1976) Studies on the structural and functional organization of system II of photosynthesis. The use of trypsin as a structurally selective inhibitor at the outer surface of the thylakoid membrane. Biochim Biophys Acta 440: 287–300
Renger G, Eckert H-J and Weiss W (1983) Studies on the mechanism of photosynthetic oxygen formation. In: Inoue Y (eds) The Oxygen Evolving System of Photosynthesis, pp 73–82. Academic Press, Japan
Renger G and Schulze A (1985) Quantitative analysis of fluorescence induction curves in isolated chloroplasts. Photobiochem Photobiophys 9: 79–87
Renger G, Rutherford AW and Völker M (1985) Evidence for resistance of the microenvironment of the primary plastoquinone acceptor (QA −Fe2+) to mild trypsination in PS II particles. FEBS Lett 185: 243–247
Renger G (1986) Herbicide interaction with PS II: Recent developments. Physiol Veg 24: 509–521
Renger G, Hagemann R and Fromme R (1986) The susceptibility of the p-benzoquinone-mediated electron transport and atrazine binding to trypsin and its modification by CaCl2 in thylakoids and PS II membrane fragments. FEBS Lett 203: 210–214
Renger G, Hanssum B, Gleiter H, Koike H and Inoue Y (1988) Interaction of 1,4-benzoquinones with Photosystem II in thylakoids and Photosystem II membrane fragments from spinach. Biochim Biophys Acta 936: 435–446
Renger G (1992) Engergy transfer and trapping in Photosystem II. In: Barber J (ed) Topics in Photosynthesis, Vol. 11, pp 45–99. Elsevier Publishers, Amsterdam
Robinson HH and Crofts AR (1983) Kinetics of the oxidation-reduction reductions of the Photosystem II quinone acceptor complex and the pathway for deactivation. FEBS Lett 153: 221–226
Rutherford AW, Crofts AR and Inoue Y (1982) Thermoluminescence as a probe of Photosystem II photochemistry. The origin of the flash-induced glow peaks. Biochim Biophys Acta 682: 457–465
Rutherford AW, Renger G, Koike H and Inoue Y (1984) Thermoluminescence as a probe of Photosystem II. The redox and protonation states of the secondary acceptor quinone and the O2-evolving enzyme. Biochim Biophys Acta 767: 548–556
Shiozawa J, Lottspeich F and Feick R (1987) The photochemical reaction center ofChloroflexus aurantiacus is composed of two structurally similar polypeptides. Eur J Biochem 167: 595–600
Tang X-S, Fushimi K and Satoh K (1990) D1-D2 complex of the Photosystem II reaction center from spinach. Isolation and partial characterization. FEBS Lett 273: 257–260
Trebst A (1986) The topology of the plastoquinone and herbicide binding peptides of Photosystem II in the thylakoid membrane. Z Naturforsch 41c: 240–245
Trebst A (1991) A contact site between the two reaction center polypeptides of Photosystem II is involved in photoinhibition. Z Naturforsch 46c: 557–562
Vass I, Horváth G, Herczeg T and Demeter S (1981) Photosynthetic energy conservation investigated by thermoluminescence. Activation energies and half-lives of thermoluminescence bands of chloroplasts determined by mathematical resolution of glow curves. Biochim Biophys Acta 634: 140–152
Vass I and Inoue Y (1986) pH dependent stabilization of S2QA − and S2OB − charge pairs studied by thermoluminescence. Photosynth Res 10: 431–436
Vermaas WFJ, Renger G and Dohnt G (1984) The reduction of the oxygen-evolving system in chloroplasts by thylakoid components. Biochim Biophys Acta 764: 194–202
Völker M, Ono T, Inoue Y and Renger G (1985) Effect of trypsin on PS II particles. Correlation between Hill activity, Mn-abundance and peptide pattern. Biochim Biophys Acta 806: 25–34
Völker M, Renger G and Rutherford AW (1986) Effects of trypsin upon EPR signals arising from components of the donor side of Photosystem II. Biochim Biophys Acta 857: 424–430
Weiss W and Renger G (1984) Analysis of the system II reaction pattern by UV-absorption changes in Tris-washed chloroplasts. In: Sybesma C (ed) Advances in Photosynthesis Research, Vol. 1, pp 167–170. Martinus Nijhoff/Dr W. Junk Publishers, The Hague
Zimmermann J-L and Rutherford AW (1986) Photoreductant-induced oxidation of Fe2+ in the electron-acceptor complex of Photosystem II. Biochim Biophys Acta 851: 416–423
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gleiter, H.M., Haag, E., Inoue, Y. et al. Functional characterisation of a purified homogeneous Photosystem II core complex with high oxygen evolution capacity from spinach. Photosynth Res 35, 41–53 (1993). https://doi.org/10.1007/BF02185410
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02185410