Abstract
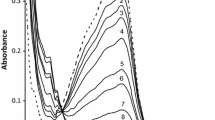
Quinoxaline-2-carboxalideneglycine complexes of iron(III), cobalt(II), nickel(II) and copper(II) complexes were subjected to a systematic TG/DTG analysis. The decomposition process consists of two stages for all these complexes. Kinetic parameters were evaluated for each of these stages using the Coats-Redfern equation. The rate of decomposition at the second stage seems to have a bearing on the catalytic effect of the metal oxides formed during this stage.
Abstract
Комплексы хиноксалин-2-карбоксалиденглицина с железом(III) ковальтом(II), никелем(II) и медью(II) были подвергнуты систематическому анализу ТГ/ДТГ. Процесс разложения для всех комплексов состоит из двух стадий. На основе уравнения Котс-Рэдферна были рассчитаны кинетические параметры каждой ступени. Скорость разложения на второй стадии, по-видимому, оказывает влияние на каталитические свойства окислов металла, образующихся на этой стадии.
Similar content being viewed by others
References
A.E. Martell, M. Calvin: Chemistry of the Metal Chelate Compounds, Prentice-Hall, Inc. Englewood Cliffs, New Jersey 1952.
K.K.M. Yusuff, R. Sreekala: Thermochim. Acta,159, 357 (1990).
K.K.M. Yusuff, R. Sreekala: Thermochim. Acta,179, 313 (1991).
K.K.M. Yusuff, R. Sreekala: Synth. React. Inorg. Met.-Org. Chem.,21, 553 (1991).
A.W. Coats, J.P. Redfern: Nature,68, 201 (1964).
K.K.M. Yusuff, A.R. Karthikeyan: Thermochim. Acta, in press.
E.S. Freeman, B. Carroll: J. Phys. Chem.,62, 394 (1958).
H.H. Horowitz, G. Metzger: Anal. Chem.,35, 1464 (1963).
J.R. Maccallum, J. Tanner: Eur. Polym. J.,6, 1033 (1970).
G.N. Natu, S.B. Kulkarni, P.S. Dhar: J. Therm. Anal.,23, 101 (1982).
D. Blecic, Z.D. Zivkovic: Thermochim. Acta,60, 68 (1983).
A.C. Norris, M.I. Pope, M. Selwood: Thermochim. Acta,41, 357 (1980).
P.M. Madhusudanan, K.K.M. Yusuff, C.G.R. Nair: J. Therm. Anal.,8, 31 (1975).
F.A. Cotton, G. Wilkinson: Advanced Inorganic Chemistry. Wiley Interscience, New York 1988.
G.C. Bond: Heterogeneous Catalysis: Principles and Applications. Oxford University Press, London 1974.
A. Clark: The Theory of Adsorption and Catalysis. Academic Press, New York 1970.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sreekala, R., Mohammed Yusuff, K.K. Thermal decomposition kinetics of iron(III), cobalt(II), nickel(II) and copper(II) complexes of a Schiff base derived from quinoxaline-2-carboxaldehyde and glycine. React Kinet Catal Lett 48, 575–581 (1992). https://doi.org/10.1007/BF02162710
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02162710