Abstract
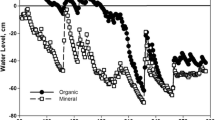
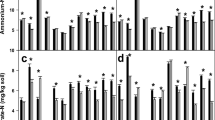
Seasonal changes in soil water and nitrogen availability were related to the phenology and growth of plants in California annual grassland. Plant accumulation of nitrogen was mainly confined to two short periods of the year: fall and early spring. At these times, plants were in the vegetative growth phase, roots were growing rapidly and soil moisture was high. During these periods, soil nitrate was low or depleted. High flux of nitrogen in this ecosystem, however, is indicated by the rapid disappearance of the previous year's detrital material, high microbial biomass, and high mineralizable nitrogen and nitrification potential.
At the end of the summer drought, significant amounts of the previous year's detrital material had disappeared, chloroform-labile N (expressed as microbial biomass N) was at its seasonal maximum, and soil inorganic nitrogen pools were high. This suggests inorganic nitrogen flux during the drought period. The ‘drought escaper’ life history characteristics of annual grasses in California annual grassland, however, may prevent plants from utilizing available nitrogen during a large part of the year.
Similar content being viewed by others
References
Alpert P, Newell E A, Chu C, Glyphis J, Gulmon S L, Hollinger D Y, Johnson N D, Mooney H A and Puttick G 1985 Allocation to reproduction in the chaparral shrubDiplacus aurantiacus. Oecologia 66, 309–316.
Belser L W and Mays E L 1980 Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Appl. Environ. Microbiol. 39, 505–510.
Bottner P 1985 Response of microbial biomass to alternate moist and dry conditions in a soil incubated with14C- and15N-labelled plant material. Soil Biol. Biochem. 17, 329–337.
Campbell C A and Davidson H R 1979 Effect of temperature, nitrogen fertilization and moisture stress on growth, assimilate distribution and moisture use by spring wheat. Can. J. Plant Sci. 69, 603–626.
Chapin F S 1980 The mineral nutrition of wild plants. Annu. Rev. Ecology and Systematics 11, 233–260.
Clark F E 1977 The internal cycling of15nitrogen in short grass prairie. Ecology 58, 1322–1333.
Davis S D and Mooney H A 1985 Comparative water relations of adjacent California shrub and grassland communities. Oecologie 66, 522–529.
Elliot E T, Horton K, Moore J C, Coleman D C and Cole C V 1984 Mineralization dynamics in fallow-dryland wheat plots, Colorado. Plant and Soil 76, 149–155.
Ewing A L and Menke J W 1983a Reproductive potential ofBromus mollis andAvena barbata under drought conditions. Madrono 30, 159–167.
Ewing A L and Menke J W 1983b Response of soft chess (Bromus mollis) and slender oat (Avena barbata) to simulated drought cycles. J. Range Management 36, 415–418.
Gulmon S L 1979 Competition and coexistence: Three annual grass species. Am. Midland Naturalist 101, 403–416.
Gutshick V P 1981 Evolved strategies in nitrogen acquisition by plants. Am. Naturalist 118, 607–637.
Jackson L E and Roy J 1986 Growth patterns of Mediterranean annual and perennial grasses under simulated rainfall regimes in southern France and California. Oecol. Plant. 7, 191–212.
Jackson L E, Houpis J L and Diemer M W 1987 The role of leaf position in the ecophysiology of an annual grass during reproductive growth. Am. Midland Naturalist (In press).
Jansson S L 1958 Tracer studies on nitrogen transformation in soil with special attention to mineralization-immobilization relationships. Ann. Royal Agriculture College, Sweden 24, 101–361.
Jenkinson D S and Powlson D S 1976 The effect of biocidal treatments on metabolism in soil. V. A method for measuring soil biomass. Soil Biol. Biochem. 8, 209–213.
Johnson D W and Edwards N T 1979 The effects of stem girdling on biochemical cycles within a mixed deciduous forest in eastern Tennessee. II. Soil nitrogen mineralization and nitrification rates. Oecologia 40, 259–271.
Jones J M and Richards B N 1977 Effect of reforestation on turnover of15N-labeled nitrate and ammonium in relation to changes in soil microflora. Soil Biol. Biochem. 9, 383–392.
Keeney D R and Nelson D W 1982 Nitrogen inorganic forms.In Methods of Soil Analysis Part 2. Eds. A L Page, R H Miller and D R Keeney. Agron. Monogr. 9 (2nd edition). ASA and SSSA Publisher, Madison, Wisconsin.
Kieft T L, Soroker E and Firestone M K 1987 Microbial biomass response to a rapid increase in water potential when dry soil is wetted. Soil. Biol. Biochem. 19, 119–126.
Munz P A 1973 A California Flora and Supplement. Univ. of California Press, Berkeley, California, 1905 p.
Page A L, Miller R H and Keeney D R 1982 Methods of Soil Analysis Part 2. Agron Monogr. 9 (2nd edition). ASA and SSSA Publisher, Madison, Wisconsin.
Parker L W, Santos P F, Phillips J and Whitford W G 1984 Carbon and nitrogen dynamics during the decomposition of litter and roots of a Chihuahuan desert annual,Lepidium lasiocarpum. Ecological Monogr. 54, 339–360.
Paul E A 1976 Nitrogen cycling in terrestrial ecosystems.In Environmental Biogeochemistry, vol. 1. Ed. J O Nriagu. pp 225–243. Ann Arbor Science Pubications Inc., Ann Arbor, Michigan.
Richards L E 1949 Methods of measuring soil moisture tension. Soil Sci. 68, 95–112.
Schultz A M, Launchbaugh J L and Biswell H H 1955 Relationship between grass density and brush seedling survival. Ecology 36, 226–238.
Schulze E-D 1983a Root-shoot interactions and plant life forms. Neth. J. Agric. Sci. 31, 291–303.
Schulze E-D 1983b Carbohydrate partitioning in relation to whole plant production and water use ofVigna unguiculata (L.) Walp. Oecologia 58, 169–177.
Vitousek P M and Melillo J M 1979 Nitrate losses from disturbed forests: Patterns and mechanisms. Forest Sci. 25, 605–619.
Voroney R P and Paul E A 1984 Determination of Kc and Kn in situ for calibration of the chloroform-fumigation incubation method. Soil Biol. Biochem. 16, 9–14.
Waring S A and Bremmer J M 1964 Ammonium production in soil under waterlogged conditions as an index of nitrogen availability to forest trees. Soil Sci. Soc. Am. J. 44, 1314–1320.
Woodmansee R G and Duncan D A 1980 Nitrogen and phosphorus dynamics and budgets in annual grasslands. Ecology 61, 893–904.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jackson, L.E., Strauss, R.B., Firestone, M.K. et al. Plant and soil nitrogen dynamics in California annual grassland. Plant Soil 110, 9–17 (1988). https://doi.org/10.1007/BF02143533
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02143533