Abstract
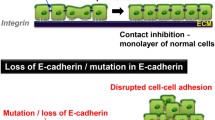
The cell adhesion molecule E-cadherin is expressed on the basolateral surfaces of normal mammary epithelial cells and is lost in a subset of breast cancers. Loss of E-cadherin expression has been postulated to facilitate tumor cell detachment from a primary tumor ultimately leading to metastasis. In this paper, I review the publishedin vitro data that initially supported this “invasion suppressor” role for E-cadherin as well as more recentin vitro andin vivo data showing that E-cadherin-positive tumor cells can metastasize. I examine other molecules required for E-cadherin function and discuss how defects in the expression or function of these molecules might alter E-cadherin function in E-cadherin-positive tumor cells. For example, loss of expression or function of catenins, intracellular molecules that interact with E-cadherin, can result in the loss of E-cadherin-mediated adhesion and a more invasive phenotype. Altered phosphorylation of E-cadherin or catenins can also influence E-cadherin function. Finally, expression of other cell surface molecules such as mucins may interfere with E-cadherin function. The collective effect of these molecules on the adhesive phenotype of breast cancer cells may be one determinant of metastatic potential.
Similar content being viewed by others
References
L. A. Liotta and E. Kohn (1990). Cancer invasion and metastasis.JAMA 263:1123–1126.
K. Boller, D. Vestweber, and R. Kemler (1985). Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells.J. Cell Biol. 100:327–332.
R. Kemler, M. Ozawa, and M. Ringwald (1989). Calcium-dependent cell adhesion molecules.Curr. Opin. Cell Biol. 1:892–897.
D. R. Garrod (1993). Desmosomes and hemidesmosomes.Curr. Opin. Cell Biol. 5:30–40.
P. Cowin (1994). Unraveling the cytoplasmic interactions of the cadherin superfamily.Proc. Natl. Acad. Sci. U.S.A. 91:10759–10761.
U. H. Frixen, J. Behrens, M. Sachs, G. Eberle, B. Voss, A. Warda, D. Lochner, and W. Birchmeier (1991). E-cadherin-mediated cell-cell adhesion prevents invasiveness.J. Cell Biol. 113:173–185.
K. Vleminckx, L. Vakaet, M. Mareel, W. Fiers, and F. van Roy (1991). Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role.Cell 66:107–119.
W. C. Chen and B. Obrink (1991). Cell-cell contacts mediated by E-cadherin (uvomorulin) restrict invasive behavior of L-cells.J. Cell Biol. 114:319–327.
M. Takeichi (1991). Cadherin cell adhesion receptors as a morphogenetic regulator.Science 251:1451–1455.
R. Kemler (1992). Classical cadherins.Sem. Cell Biol. 3:149–155.
B. Geiger and O. Ayalon (1992). Cadherins.Annu. Rev. Cell Biol. 8:307–332.
S. Suzuki, K. Sano, and H. Tanihara (1991). Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue.Cell Reg. 2:261–270.
C. W. Liaw, C. Cannon, M. D. Power, P. K. Kiboneka, and L. L. Rubin (1990). Identification and cloning of two species of cadherins in bovine endothelial cells.EMBO J. 9:2701–2708.
Y.-Y. Xiang, M. Tanaka, M. Suzuki, H. Igarashi, E. Kiyokawa, Y. Naito, Y. Ohtawara, Q. Shen, H. Sugimura, and E. Kino (1994). Isolation of complementary DNA encoding K-cadherin, a novel rat cadherin preferentially expressed in fetal kidney and kidney carcinoma.Cancer Res. 54:3034–3041.
J. Palacios, N. Benito, A. Pizarro, A. Suarez, J. Espada, A. Cano, and C. Gamallo (1995). Anomalous expression of P-cadherin in breast carcinoma: correlation with E-cadherin expression and pathological features.Am. J. Pathol. 146:605–612.
A. Nagafuchi and M. Takeichi (1988). Cell binding function of E-cadherin is regulated by the cytoplasmic domain.EMBO J. 7:3679–3684.
M. Ozawa, H. Baribault, and R. Kemler (1989). The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species.EMBO J. 8:1711–1717.
A. Nagafuchi, M. Takeichi, and S. Tsukita (1989). The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression.Cell 65:849–857.
P. D. McCrea, C. W. Turck, and B. Gumbiner (1991). A homolog of the armadillo protein in Drosophila (plakoglobin) associates with E-cadherin.Science 254:1359–1361.
S. Butz, J. Stappert, H. Weissig, and R. Kemler (1992). Plakoglobin and beta-catenin: distinct but closely related [letter].Science 257:1142–1144.
M. Peifer, P. D. McCrea, K. J. Green, E. Wieschaus, and B. M. Gumbiner (1992). The vertebrate adhesive junction proteins beta-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properites.J. Cell Biol. 118:681–691.
K. A. Knudsen and M. J. Wheelock (1992). Plakoglobin, or an 83-kD homologue distinct from beta-catenin, interacts with E-cadherin and N-cadherin.J. Cell Biol. 118:671–679.
D. F. Agbib and P. D. McCrea (1995). The E-cadherin complex contains the src substrate p120.Exp. Cell Res. 218:359–369.
A. B. Reynolds, L. Herbert, J. L. Cleveland, S. T. Berg, and J. R. Gaut (1992). p120, a novel substrate of protein tyrosine kinase receptors and of p60v-src, is related to cadherin-binding factors beta-catenin, plakoglobin and armadillo.Oncogene 7:2439–2445.
A. B. Reynolds, J. Daniel, P. D. McCrea, M. J. Wheelock, J. Wu, and Z. Zhang (1994). Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes.Mol. Cell Biol. 14:8333–8342.
S. Shibamoto, M. Hayakawa, K. Takeuchi, T. Hori, K. Miyazawa, N. Kitamura, K. R. Johnson, M. J. Wheelock, N. Matsuyoshi, M. Takeichi, and F. Ito. Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes.J. Cell Biol. 128:949–957.
T.-S. Jou, D. B. Stewart, J. Stappert, W. J. Nelson, and J. A. Marrs (1995). Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex.Proc. Natl. Acad. Sci. U.S.A. 92:5067–5071.
J. Hulsken, W. Birchmeier, and J. Behrens (1994). E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton.J. Cell Biol. 127:2061–2069.
S. Butz and R. Kemler (1994). Distinct cadherin-catenin complexes in Ca2+-dependent cell-cell adhesion.FEBS Lett. 355:195–200.
K. A. Knudsen, A. Peralta Soler, K. R. Johnson, and M. J. Wheelock (1995). Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin.J. Cell Biol. 130:67–77.
J. E. Darnell, H. F. Lodish, and D. Baltimore (1990).Molecular Cell Biology, Scientific American Books, Inc., New York, pp. 878–881.
Y. Shimoyama, A. Nagafuchi, S. Fujita, M. Gotoh, M. Takeichi, S. Tsukita, and S. Hirohashi (1992). Cadherin dysfunction in a human cancer cell line: possible involvement of loss of alphacatenin expression in reduced cell-cell adhesiveness.Cancer Res. 52:5770–5774.
S. Hirano, N. Kimoto, Y. Shimoyama, S. Hirohashi, and M. Takeichi (1992). Identification of a neural alpha-catenin as a key regulator of cadherin function and multicellular organization.Cell 70:293–301.
M. Watabe, A. Nagafuchi, S. Tsukita, and M. Takeichi (1994). Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line.J. Cell Biol. 127:247–256.
T. Oyama, Y. Kanai, A. Ochiai, S. Akimoto, T. Oda, K. Yanagihara, A. Nagafuchi, S. Tsukita, S. Shibamoto, F. Ito, M. Takeichi, H. Matsuda, and S. Hirohashi (1994). A truncated betacatenin disrupts the interaction between E-cadherin and alphacatenin: a cause of loss of intercellular adhesiveness in human cancer cell lines.Cancer Res. 54:6282–6287.
J. Kawanishi, J. Kato, K. Sasaki, S. Fujii, N. Watanabe, and Y. Niitsu (1995). Loss of E-cadherin-dependent cell-cell adhesion due to mutation of the beta-catenin gene in a human cancer cell line, HSC-39.Mol. Cell Biol. 15:1175–1181.
E. Breen, A. Clarke, G. Steele Jr., and A. M. Mercurio (1993). Poorly differentiated colon carcinoma cell lines deficient in alpha-catenin expression express high levels of surface E-cadherin but lack calcium-dependent cell-cell adhesion.Cell Adhes. Commun. 1:239–250.
R. A. Morton, C. M. Ewing, A. Nagafuchi, S. Tsukita, and W. B. Isaacs (1993). Reduction of E-cadherin levels and deletion of the alpha-catenin gene in human prostate cancer cells.Cancer Res. 53:3585–3590.
B. Rubinfeld, B. Souza, I. Albert, O. Muller, S. H. Chamberlain, F. R. Masiarz, S. Munemitsu, and P. Polakis (1993). Association of the APC gene product with beta-catenin.Science 262:1731–1734.
L.-K. Su, B. Vogelstein, and K. Kinzler (1993). Association of the APC tumor suppressor protein with catenins.Science 262:1734–1737.
B. Rubinfeld, B. Souza, I. Albert, S. Munemitsu, and P. Polakis (1995). The APC protein and E-cadherin form similar but independent complexes with alpha-catenin, beta-catenin, and plakoglobin.J. Biol. Chem. 270:5549–5555.
S. Munemitsu, I. Albert, B. Souza, B. Rubinfeld, and P. Polakis (1995). Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein.Proc. Natl. Acad. Sci. U.S.A. 92:3046–3050.
C. L. Sommers, S. W. Byers, E. W. Thompson, J. A. Torri, and E. P. Gelmann (1994). Differentiation state and invasiveness of human breast cancer cell lines.Breast Cancer Res. Treat. 31:325–335.
E. W. Thompson, S. Paik, N. Brunner, C. L. Sommers, G. Zugmaier, R. Clarke, T. B. Shima, J. Torri, S. Donohue, M. E. Lippman, G. R. Martin, and R. B. Dickson (1992). Association of increased basement membrane-invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines.J. Cell Physiol. 150:534–544.
C. L. Sommers, E. W. Thompson, J. A. Torri, R. Kemler, E. P. Gelmann, and S. W. Byers (1991). Cell adhesion molecule uvomorulin expression in human breast cancer cell lines: relationship to morphology and invasive capacities.Cell Growth Diff. 2:365–372.
C. L. Sommers, E. P. Gelmann, R. Kemler, P. Cowin, and S. W. Byers (1994). Alterations in beta-catenin phosphorylation and plakoglobin expression in human breast cancer cells.Cancer Res. 54:3544–3552.
S. W. Byers, C. L. Sommers, B. Hoxter, A. M. Mercurio, and A. Tozeren (1995). Role of E-cadherin in the response of tumor cell aggregates to lymphatic venous and arterial flow: measurement of cell-cell adhesion strength.J. Cell Sci. 108:2053–2064.
P. Navarro, E. Lozano, and A. Cano (1993). Expression of E- or P-cadherin is not sufficient to modify the morphology and the tumorigenic behavior of murine spindle carcinoma cells.J. Cell Sci. 105:923–934.
M. T. Dimanche-Boitrel, L. Vakaet, P. Pujuguet, B. Chauffert, M. S. Martin, A. Hammann, F. van Roy, M. Mareel, and F. Martin (1994).In vivo andin vitro invasiveness of a rat colon cancer cell line maintaining E-cadherin expression: an enhancing role of tumor-associated myofibroblasts.Int. J. Cancer 56:512–521.
H. Oka, H. Shiozaki, K. Kobayashi, M. Inoue, H. Tahara, T. Kobayashi, M. Takeichi, and T. Mori (1993). Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis.Cancer Res. 53:1696–1701.
C. Gamallo, J. Palacios, A. Suarez, A. Pizarro, P. Navarro, M. Quintanilla, and A. Cano (1993). Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma.Am. J. Pathol. 142:987–993.
S. A. Rasbridge, C. E. Gillett, S. A. Sampson, F. S. Walsh, and R. R. Millis (1993). Epithelial (E-) and placental (P-) cadherin cell adhesion molecule expression in breast carcinoma.J. Pathol. 169:245–250.
P. Lipponen, E. Saarelainen, H. Ji, S. Aaltomaa, and K. Syrjanen (1994). Expression of E-cadherin (E-cd) as related to other prognostic factors and survival in breast cancer.J. Pathol. 174:101–109.
R. Moll, M. Mitze, U. H. Frixen, and W. Birchmeier (1993). Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas.Am. J. Pathol. 143:1731–1742.
D. L. Rimm, J. H. Sinard, and J. Morrow (1995). Reduced alpha-catenin and E-cadherin expression in breast cancer.Lab. Invest. 72:506–512.
E. Reichmann, H. Schwarz, E. M. Deiner, I. Leitner, M. Eilers, J. Berger, M. Busslinger, and H. Beug (1992). Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion.Cell 71:1103–1116.
D. Alford and J. Taylor-Papadimitriou (1996). Cell adhesion molecules in the normal and cancerous mammary gland.J. Mam. Gland Biol. Neoplasia 1(2) 207–218.
B. D'Souza and J. Taylor-Papadimitriou (1994). Overexpression of ERBB2 in human mammary epithelial cells signals inhibition of transcription of the E-cadherin gene.Proc. Natl. Acad. Sci. U.S.A. 91:7202–7206.
P. J. Lu, Q. L. Lu, A. Rughetti, and J. Taylor-Papadimitriou (1995). bcl-2 overexpression inhibits cell death and promotes the morphogenesis, but not tumorigenesis of human mammary epithelial cells.J. Cell Biol. 129:1363–1378.
T. Oda, Y. Kanai, T. Oyama, K. Yoshiura, Y. Shimoyama, W. Birchmeier, T. Sugimura, and S. Hirohashi (1994). E-cadherin gene mutations in human gastric carcinoma cell lines.Proc. Natl. Acad. Sci. U.S.A. 91:1858–1862.
K. F. Becker, M. J. Atkinson, U. Reich, I. Becker, H. Nekarda, J. R. Siewert, and H. Hofler (1994). E-cadherin gene mutations provide clues to diffuse type gastric carcinomas.Cancer Res. 54:3845–3852.
Y. Kanai, T. Oda, H. Tsuda, A. Ochiai, and S. Hirohashi (1994). Point mutation of the E-cadherin gene in invasive lobular carcinoma of the breast.Jpn. J. Cancer Res. 85:1035–1039.
M. Ozawa, J. Engel, and R. Kemler (1990). Single amino acid substitutions in one Ca2+ binding site of uvomorulin abolish the adhesive function.Cell 63:1033–1038.
C. H. Damsky, J. Richa, D. Solter, K. Knudsen, and C. A. Buck (1983). Identification of purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue.Cell 34:455–466.
M. Katayama, S. Hirai, K. Kamihagi, K. Nakagawa, M. Yasumoto, and I. Kato (1994). Soluble E-cadherin fragments increased in circulation of cancer patients.Br. J. Cancer 69:580–585.
N. Matsuyoshi, T. Tanaka, K. Toda, H. Okamoto, F. Furukawa, and S. Imamura (1995). Soluble E-cadherin: a novel cutaneous disease marker.Br. J. Dermatol. 132:745–749.
M. J. Wheelock, C. A. Buck, K. B. Bechtol, and C. H. Damsky (1987). Soluble 80-kd fragment of cell-CAM 120/80 disrupts cell-cell adhesion.J. Cell Biochem. 34:187–202.
K. L. Vleminckx, J. J. Deman, E. A. Bruyneel, G. M. R. Vandenbossche, A. A. Keirsebilck, M. M. Mareel, and F. M. van Roy (1994). Enlarged cell-associated proteoglycans abolish E-cadherin functionality in invasive tumor cells.Cancer Res. 54:873–877.
J. Hilkens, H. L. Vos, J. Wesseling, M. Boer, J. Storm, S. van der Valk, J. Calafat, and C. Patriarca (1995). Is episialin/MUC1 involved in breast cancer progression?Cancer Lett. 90:27–33.
M. J. L. Ligtenberg, F. Buijs, H. L. Vos, and J. Hilkens (1992). Suppression of cellular aggregation by high levels of episialin.Cancer Res. 52:2318–2324.
H. Kemperman, Y. Wijnands, J. Wesseling, C. M. Niessen, A. Sonnenberg, and E. Roos (1994). The mucin epiglycanin on TA3/Ha carcinoma cells prevents alpha6 beta4-mediated adhesion to laminin and kalinin and E-cadherin-mediated cell-cell interaction.J. Cell Biol. 127:2071–2080.
N. Matsuyoshi, M. Hamaguchi, S. Taniguchi, A. Nagafuchi, S. Tsukita, and M. Takeichi (1992). Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts.J. Cell Biol. 118:703–714.
J. Behrens, L. Vakaet, R. Friis, E. Winterhager, F. Van Roy, M. M. Mareel, and W. Birchmeier (1993). Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene.J. Cell Biol. 120:757–766.
S. Shibamoto, M. Hayakawa, K. Takeuchi, T. Hori, N. Oku, K. Miyazawa, N. Kitamura, M. Takeichi, and F. Ito (1994). Tyrosine phosphorylation of beta-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells.Cell Adhes. Commun. 1:295–305.
J. Stappert and R. Kemler (1994). A short core region of Ecadherin is essential for catenin binding and is highly phosphorylated.Cell Adhes. Commun. 2:319–327.
M. A. Anzano, S. W. Byers, J. M. Smith, C. W. Peer, L. T. Mullen, C. C. Brown, A. B. Roberts, and M. B. Sporn (1994). Prevention of breast cancer in the rat with 9-cis-retinoic acid as a single agent and in combination with tamoxifen.Cancer Res. 54:4614–4617.
S. W. Byers (submitted).
C. W. Daniel, P. Strickland, and Y. Friedmann (1995). Expression and functional role of E- and P-cadherins in mouse mammary ductal morphogenesis and growth.Dev. Biol. 169:511–519.
S. Fujioka, N. Kohno, and K. Hiwada (1995). Ubenimex activates the E-cadherin-mediated adhesion of a breast cancer cell line YMB-S.Jpn. J. Cancer Res. 86:368–373.
M. E. Bracke, C. Charlier, E. A. Bruyneel, C. Labit, M. M. Mareel, and V. Castronovo (1994). Tamoxifen restores the E-cadherin function in human breast cancer MCF-7/6 cells and suppresses their invasive phenotype.Cancer Res. 54:4607–4609.
M. Overduin, T. S. Harvey, S. Bagby, K. I. Tong, P. Yau, M. Takeichi, and M. Ikura (1995). Solution structure of the epithelial cadherin domain responsible for selective cell adhesion.Science 267:386–389.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sommers, C.L. The role of cadherin-mediated adhesion in breast cancer. J Mammary Gland Biol Neoplasia 1, 219–229 (1996). https://doi.org/10.1007/BF02013645
Issue Date:
DOI: https://doi.org/10.1007/BF02013645