Abstract
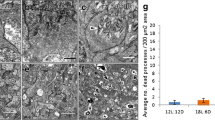
Male albino (Sprague Dawley) and pigmented (Norwegian Brown) rats received 1% 2,5-hexanediol (H) in their drinking water for 5 or 8 weeks, respectively. The rats were housed either in 12 h light (average 30 cd/cm2 inside cage) and 12 h darkness (group LH) or in total darkness (group DH). Two control groups (Light only, LC; Darkness only, DC) were studied in parallel under identical conditions. The animals were sacrificed at the end of H exposure or after an ensuing 13-week period without H but under the same lighting conditions. The retinas of albino rats in the LH group showed a reduction (compared to the LC, DH and DC groups) in the number of nuclei per unit area of the outer nuclear layer (ONL;p<0.05) and degeneration of the outer segment and the inner segment layers (photoreceptor cells). A less pronounced loss of nuclei was seen in the LC group. No decrease in the number of nuclei, or signs of degeneration, were demonstrated in the albino DH or DC groups. Thirteen weeks after exposure to H, the albino LH rats had lost about 50% of the nuclei in the ONL (p<0.05) and the outer plexiform layer (OPL) had almost disappeared. At the corresponding time, in the pigmented rats the LH and DH groups differed from the LC and DC groups. The degenerative process resulted in no inflammatory changes in the retina. The results imply an interaction exceeding simple summation after exposure to light and H, in destroying photoreceptors and OPL (p<0.001) in albino rats. The morphological damage progresses even after the removal of H from the diet. While pigmented rats are susceptible to retinal damage induced by H, they seem to be less sensitive to H together with light, and also develop less severe signs of neurological dysfunction than those seen in albinos after exposure to equivalent doses of H.
Similar content being viewed by others
References
Bäckström B, Collins VP (1987) Cytoskeletal changes in axons of rats exposed to 2,5-hexanediol, demonstrated using monoclonal antibodies. Neurotoxicology 8: 85–96
Bäckström B, Dumanski JP, Collins VP (1990) The effects of 2,5-hexanedione on the retina of albino rats. Neurotoxicology 11: 47–55
Blain L, Mergler D (1986) La dyschromatopsie chez des personnes exposées professionnellement aux solvants organiques. J Fr Ophtalmol 9: 127–133
Cavanagh JB (1982a) The pattern of recovery of axons in the nervous system of rats following 2,5-hexanediol intoxication: a question of rheology? Neuropathol Appl Neurobiol 8: 19–34
Cavanagh JB (1982b) The pathokinetics of acrylamide intoxication: a reassessment of the problem. Neuropathol Appl Neurobiol 8: 315–336
Cavanagh JB, Bennetts RJ (1981) On the pattern of changes in the rat nervous system produced by 2,5-hexanediol. A topographical study by light microscopy. Brain 104: 297–318
Chang YC (1990) Patients withn-hexane induced polyneuropathy: a clinical follow up. Br J Ind Med 47: 485–489
Cody RP, Smith JK (1987) Applied statistics and the SAS programming language, 2nd edn. Elsevier Science Publishing Co. Inc., New York
Colombi A, Maroni M, Picchi O, Rota E, Castano P, Foa V (1981) Carbon disulfide neuropathy in rats. A morphological and ultrastructural study of degeneration and regeneration. Clin Toxicol 18: 1463–1474
Eskin TA, Lapham LW, Maurissen JPJ, Merigan WH (1985) Acrylamide effects on the macaque visual system. II. Retinogeniculate morphology. Invest Ophthalmol Vis Sci 26: 317–329
Eskin TA, Merigan WH, Wood RW (1988) Carbon disulfide effects on the visual system. II. Retinogeniculate degeneration. Invest Ophthalmol Vis Sci 29: 519–527
Fox DA, Chu LW (1988) Rods are selectively altered by lead: II. Ultrastructure and quantitative histology. Exp Eye Res 46: 613–625
Jones HB, Cavanagh JB (1982a) Recovery from 2,5-hexanediol intoxication of the retinotectal tract of the rat. An ultrastructural study. Acta Neuropathol 58: 286–290
Jones HB, Cavanagh JB (1982b) The early evolution of neurofilamentous accumulations due to 2,5-hexanediol in the optic pathways of the rat. Neuropathol Appl Neurobiol 8: 289–301
Lynch III JJ, Merigan WH, Eskin TA (1989) Subchronic dosing macaques with 2,5-hexanedione causes long-lasting motor dysfunction but reversible visual loss. Toxicol Appl Pharmacol 98: 166–180
Mergler D, Blain L (1987) Assessing color vision loss among solventexposed workers. Am J Ind Med 12: 195–203
Mergler D, Blain L, Lagacé JP (1987) Solvent related colour vision loss: an indicator of neural damage? Int Arch Occup Environ Health 59: 313–321
Noell WK, Walker VS, Kang BS, Berman S (1966) Retinal damage by light in rats. Invest Ophthalmol 5: 450–473
Nylén P, Bäckström B, Hagman M, Johnson A-C, Collins VP, Höglund G (1993) Effect of exposure to 2,5-hexanediol in light or darkness on the retina of albino and pigmented rats. II. Electrophysiological studies. Arch Toxicol (in press)
Pasternak T, Flood DG, Eskin TA, Merigan WH (1985) Selective damage to large cells in the cat retinogeniculate pathway by 2,5-hexanedione. J Neurosci 5: 1641–1652
Powell HC, Koch T, Garrett R, Lampert PW (1978) Schwann cell abnormalities in 2,5-hexanedione neuropathy. J Neurocytol 7: 517–528
Raitta C, Seppäläinen AM, Huuskonen MS (1978)n-Hexane maculopathy in industrial workers. Albrecht von Graefes Arch Clin Exp Ophthalmol 209: 99–110
Rapp LM, Williams TP (1980) The role of ocular pigmentation in protecting against retinal light damage. Vision Res: 1127–1131
Sheehan DC, Hrapchak BB (1980) Theory and practice of histotechnology, 2nd edn. C. V. Mosby Company, St Louis, Toronto, London, pp 40–58
Spencer PS, Schaumburg HH (1975) Experimental neuropathy produced by 2,5-hexanedione — a major metabolite of the neurotoxic industrial solvent methyl n-butylketone. J Neurol Neurosurg Psychiatry 38: 771–775
Spencer PS, Schaumburg HH, Sabri MI, Veronesi B (1980) The enlarging view of hexacarbon neurotoxicity. CRC Crit Rev Toxicol 7: 279–356
Sperling HG, Johnson C, Harwerth RS (1980) Differential spectral photic damage to primate cones. Vision Res 20: 1117–1125
Teir H (1988) An ophthalmological study on workers with long-term occupational exposure to industrial solvents. Academic dissertation, Department of Ophthalmology, University of Helsinki, Helsinki, ISBN 952-90028-0-7
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bäckström, B., Nylén, P., Hagman, M. et al. Effect of exposure to 2,5-hexanediol in light or darkness on the retina of albino and pigmented rats. I. Morphology. Arch Toxicol 67, 277–283 (1993). https://doi.org/10.1007/BF01974347
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01974347