Abstract
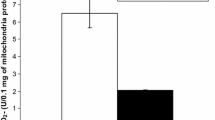
The lung toxicity of a carbide-cobalt mixture is more important than that of each individual component; the mechanism of this interaction is not understood. The capacity of cobalt metal particles alone and mixed with different carbides to generate hydroxyl radicals was examined with the deoxyribose assay. In a chemical system, cobalt ions and cobalt metal particles (Co) were found to catalyse the degradation of deoxyribose in the presence of hydrogen peroxide. Carbides were able to directly oxidize deoxyribose, but their respective activities did not support such a mechanism to explain the carbide-cobalt interactive toxicity, since there was no direct relationship between deoxyribose degradation ability and cytotoxicity toward macrophages. Tungsten, niobium, titanium and chromium carbides (interactive carbides) were only weak oxidants and conversely molybdenum, vanadium and silicon carbides (non-interactive carbides) were the most potent ones. The ability of cobalt metal to produce hydroxyl radicals in the presence of hydrogen peroxide was not increased by tungsten carbide. The role of reactive radical formation in the toxicity of these particles was further assessed in a macrophage culture model. Catalase (4000 U/ml), superoxide dismutase (300 U/ml), sodium azide (1 mM), sodium benzoate, mannitol, taurine and methionine (all 20 mM) were all unable to protect against the cytotoxic effects of cobalt ions and cobalt metal alone or mixed with tungsten carbide. In conclusion, no evidence was found that production of reactive oxygen species contributes to the elective toxicity of carbide-cobalt mixtures.
Similar content being viewed by others
References
Cugell D, Morgan W, Perkins D, Rubin A (1990) Respiratory effects of cobalt. Arch Int Med 150: 177–183
Fillat C, Rodriguez-Gil JE, Guinovart JJ (1992) Molybdate and tungstate act like vanadate on glucose metabolism in isolated hepatocytes. Biochem J 282: 659–663
Halliwell B, Gutteridge JMC (1985) Hydroxyl radicals assayed by aromatic hydroxylation and deoxyribose degradation. In: Greenwald RA (ed) Handbook of methods for oxygen radical research. CRC, Boca Raton
Hanna PM, Kadiiska MB, Mason RP (1991) Oxygen-derived free radical and active oxygen complex formation from cobalt(II) chelates in vitro. Chem Res Toxicol 5: 109–115
Kadiiska MB, Maples KR, Mason RP (1989) A comparison of cobalt(II) and iron(II) hydroxyl and Superoxide free radical formation. Arch Biochem Biophys 275: 98–111
Kennedy TP, Dodson R, Rao NV, Ky H, Hopkins C, Baser M, Tolley E, Hoidal JR (1989) Dusts causing pneumoconiosis generate ∘OH and produce hemolysis by acting as Fenton catalysts. Arch Biochem Biophys 269: 359–364
Kilinc K, Rouhani R (1992) Cobaltous ion inhibition of lipid peroxidation in biological membranes. Biochim Biophys Acta 1125: 189–195
Lasfargues G, Lison D, Maldague P, Lauwerys R (1992) Comparative study of the acute lung toxicity of pure cobalt powder and cobalt-tungsten carbide mixture in the rat. Toxicol Appl Pharmacol 112: 41–50
Lauwerys R, Lison D (1993) Health risks associated with cobalt exposure: an overview. Sci Total Environ (in press)
Lewis CPL, Demedts M, Nemery B (1991) Indices of oxidative stress in hamster lung following exposure to cobalt(II) ions: in vivo and in vitro studies. Am J Respir Cell Mol Biol 5: 163–169
Lison D, Lauwerys R (1990) In vitro cytotoxic effects of cobalt containing dusts on mouse peritoneal and rat alveolar macrophages. Environ Res 52: 187–198
Lison D, Lauwerys R (1991) Biological responses of isolated macrophages to cobalt metal and tungsten carbide-cobalt powders. Pharmacol Toxicol 69: 282–285
Lison D, Lauwerys R (1992) Study of the mechanism responsible for the elective toxicity of tungsten carbide-cobalt powder toward macrophages. Toxicol Lett 60: 203–210
Moorhouse CP, Halliwell B, Grootveld M, Gutteridge JMC (1985) Cobalt(II) ion as a promoter of hydroxyl radical and possible ‘cryptohydroxyl’ radical formation under physiological conditions. Differential effects of hydroxyl radical scavengers. Biochim Biophys Acta 843: 261–268
Ottonello L, Dallegri F, Dapino P, Pastorino G, Sachetti C (1991) Cytoprotection against neutrophil-delivered oxidant attack by antibiotics. Biochem Pharmacol 42: 2317–2321
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lison, D., Lauwerys, R. Evaluation of the role of reactive oxygen species in the interactive toxicity of carbide-cobalt mixtures on macrophages in culture. Arch Toxicol 67, 347–351 (1993). https://doi.org/10.1007/BF01973706
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01973706