Abstract
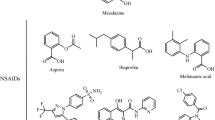
Since most non-steroidal anti-inflammatory drugs (NSAIDs) contain only one obvious ionisable group at physiological pH levels then they may be easily identified as having either acidic or basic character. Basic NSAIDs are simply non-acidic NSAIDs capable of accepting a proton within the physiological pH range. Within this range, however, a few NSAIDs contain two obvious ionisable groups, one acidic and the other basic. Such compounds should be described as amphiprotic, and include NSAIDs such as 4- and 5-amino substituted salicylic acids, niflumic acid, amfenac, WY 18251, and azapropazone. The aqueous ionisation equilibrium of such compounds is complex and is described by two macroscopic ionisation constants. Evidence has accumulated during the last decade to support the view that the pharmacokinetic behaviour of NSAIDs contributes not only decisively to their therapeutic effects but also to the type and incidence of their side-effects.A priori, using a physico-chemical argument, certain amphiprotic NSAIDs should be better tolerated by the gastric mucosa than the classical acidic compound. Of those NSAIDs commercially available in the United Kingdom azapropazone remains the only one for which amphiprotic behaviour has been described. Following our examination of available data for azapropazone we conclude that the use of amphiprotic compounds represents a logical approach towards solving the problem of NSAID-induced gastric mucosal damage.
Similar content being viewed by others
References
Brune K (1974) How aspirin might work: a pharmacokinetic approach. Agents Actions 4 (4): 230–232
Brune K (1982) Prostaglandins inflammation and anti-inflammatory drugs. Eur J Rheumatol Inflam 5 (4): 335–349
Brune K, Graf P (1978) Non-steroid anti-inflammatory drugs: influence of extra-cellular pH on biodistribution and pharmacological effects. Biochem Pharmacol 27 (4): 525–530
Brune K, Graf P, Rainsford KD (1977a) A pharmacokinetic approach to the understanding of therapeutic effects and side effects of salicylates. Agents Actions (Suppl) 1: 9–25
Brune K, Schweitzer A, Eckert H (1977b) Parietal cells of the stomach trap salicylates during absorption. Biochem Pharmacol 26 (18): 1735–1740
Brune K, Gubler H, Schweitzer A (1979) Autoradiographic methods for the evaluation of ulcerogenic effects of anit-inflammatory drugs. Pharmacol Ther 5: 199–207
Brune K, Schweitzer A, Lanz R (1984) Importance of drug biodistribution and metabolism in the development of side-effects by anti-inflammatory/analgesic drugs. Adv Inflam Res 6: 9–15
Cullen E (1984) Novel anti-inflammatory agents. J Pharm Sci 73 (5): 579–589
Fenner H, Mixich G (1973) NMR studies of the molecular structure of azapropazone and interpretation of its pharmacokinetics and biotransformation. Arzneim Forsch 23 (5): 667–669
Graf P, Glatt M, Brune K (1975) Acidic non-steroid anti-inflammatory drugs accumulating in inflamed tissue. Experientia 31 (8): 951–953
Gubler HU, Baggliolini M (1978) Pharmacological properties of proquazone. Scand J Rheumatol 21: 8–11
Herzfeldt CD, Kummel R (1983) Dissociation constants, solubilities and dissolution rates of some selected non-steroidal anti-inflammatories. Drug Dev Ind Pharm 9 (5): 767–793
Jahn V, Reller J, Schatz F (1973) Pharmacokinetic experiments with azapropazone in animals. Arzneim Forsch 13 (5): 660–666
Klatt L, Koss FW (1973) Pharmacokinetic studies with azapropazone dihydrate labelled with carbon-14, in the rat. Arzneim Forsch 23 (7): 913–919
Krebs HA, Speakman JC (1945) The effect of pH on the solubility of sulphonamides. Biochem J 39: xlii
Leonards JR (1963) The influence of solubility on the rate of gastrointestinal absorption of aspirin. Clin Pharmacol Ther 4: 476–479
Lombardino JG (1974) Enolic acids with antiinflammatory activity. Med Chem 13 (2): 130–157
Luzzani F, Colombo G, Schiatti P, Selva D, Glasser A (1984) Inhibition of PG production by MDL 305, a new non-steroidal anti-inflammatory compound, in rat gastric mucosa and inflammatory exudate. Pharmacol Res Commun 16 (8) 755–763
Marsh CC, Schuna AA, Sundstrom MD (1986) A review of selected investigational nonsteroidal anti-inflammatory drugs of the 1980s. Pharmacotherapy 6 (1): 10–25
McCormack K, Brune K (1987) Classical absorption theory and the development of gastric mucosal damage associated with the non-steroidal anti-inflammatory drugs. Arch Toxicol 60 (4): 261–269
Rainsford KD (1978) Structure-activity relationships of non-steroid anti-inflammatory drugs. Gastric ulcerogenic activity. Agents Actions 8 (6): 587–605
Rainsford KD, Brune K (1978) Selective cytotoxic actions of aspirin on parietal cells: a principal factor in the early stages of aspirin-induced gastric damage. Arch Toxicol 40 (2): 143–150
Rainsford KD, Willis C (1982) Relationship of gastric mucosal damage induced in pigs by anti-inflammatory drugs to their effects on prostaglandin production. Dig Dis Sci 27 (7): 624–635
Rainsford KD, Fox SA, Osborne DJ (1984) Comparative effects of some non-steroidal anti-inflammatory drugs on the ultra-structural integrity and prostaglandin levels in the rat gastric mucosa: relationship to drug intake. Scand J Gastroenterol 19 (101): 55–68
Schweitzer A, Brune K (1977) Salicylic acid and proquazone: the differences in absorption and biodistribution explain their different profile of side-effects. In: Willoughby DA et al. (ed) Perspectives in inflammation. MTP Press, UK, pp 353–360
Shen TY (1977) The expanding vistas of nonacidic antiarthritic agents. Drugs Exp Clin Res 2 (1): 1–8
Takesue EL, Perrine JW, Trapold JH (1976) The anti-inflammatory profile of proquazone. Arch Int Pharmacodyn 221: 122–131
Upadhyay R, Duncan A, Walker FS, Russell RI (1989) A new method for studying gastric and intestinal absorption of drugs and its application to azapropazone. Eur J Clin Pharmacol (in press)
Verbeeck RK, Blackburn JL, Loewen GR (1983) Clinical pharma-cokinetics of non-steroidal anti-inflammatory drugs. Clin Pharmacokinet 8: 297–331
Walker FS (1985) Azapropazone and related benzotriazines, In: Rainsford KD (ed) Anti-inflammatory drugs. CRC Press Inc, Florida pp 1–32
Walker FS (1987) Azapropazone is not a pyrazolidine derivative. In: Rainsford KD, Vallow CP (ed) Side effects of anti-inflammatory drugs. Part II: Studies in major organ systems. MTP press, Lancaster, pp 431–438
Whittle BJR (1986) The mechanisms of gastric damage by nonsteroid anti-inflammatory drugs. In: Cohen MM (ed) Biological protection with prostaglandins, Volume II. CRC Press Inc, Florida, pp 1–27
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McCormack, K., Brune, K. The amphiprotic character of azapropazone and its relevance to the gastric mucosa. Arch Toxicol 64, 1–6 (1990). https://doi.org/10.1007/BF01973369
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01973369