Summary
Endothelial cells covering the luminal surface of vessels are exposed to at least two different mechanical forces: 1) fluid shear stress produced by the circulation of blood, and 2) periodic stretching and relaxing as a result of the diameter oscillations caused by blood pulsation.
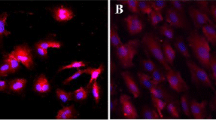
In this study we present an apparatus which was constructed to imitate the volume pulse with its typical incisura of the abdominal aorta. Using this apparatus, we exposed cultured endothelial cells to continuously produced cyclic and directional stretching and relaxation for three days. In all experiments cells remained attached and viable when subjected to mechanical stimulation. The vast majority of endothelial cells which underwent mechanical stimulation became elongated and oriented with their longer axis perpendicular to the direction of stretching (angle of cell orientation: α=88.7°±12°;\(\bar x\) ±SD), whereas cells on unstretched membranes had a cobblestone-like appearance and remained in random orientation. In the stretched cells, the factor of elongation was f=6.8±1.3;\(\bar x\) ±SD; unstretched cells which exhibited a polygonal shape had a factor of elongation of f=1.8±0.8;\(\bar x\) ±SD. In addition, the behavior of cytoskeletal components such as microfilaments and microtubules was examined in the process of cell orientation as both are actively involved in alterations of cell shape and cell migration. Actin filaments were oriented in parallel alignment perpendicular to the stretch direction (angle of actin filament orientation: β=90.4°±9°;\(\bar x\) ±SD). A distinct orientation of microtubules was not observed, althougn a noticeable number of microtubules was observed to be in parallel alignment. Furthermore, microtubules of cells which underwent mechanical stimulation exhibited a pronounced asymmetric intracellular distribution with strongly fluorescent cytoplasmic areas in which microtubules seemed to be accumulated.
The results indicate that endothelial cell elongation and orientation in vitro can be induced by periodic stretching and relaxation comparable to the periodic oscillations of the vessel wall due to blood pulsation in vivo.
Similar content being viewed by others
References
Buck RC (1980) Reorientation response of cells to repeated stretch and recoil of the substratum. Exp Cell Res 127:470–474
Buck RC (1982) The influence of contact guidance on the orientation of colonies of subcultured vascular smooth muscle cells. In Vitro 18:783–788
Buck RC (1983) Behaviour of vascular smooth muscle cells during repeated stretching of the substratum in vitro. Atherosclerosis 46:217–223
Bussolari SR, Dewey CF Jr, Gimbrone MA Jr (1982) Apparatus for subjecting living cells to fluid shear stress. Rev Sci Instrum 53:1851–1854
Brunette DM (1986) Spreading and orientation of epithelial cells on grooved substrata. Exp Cell Res 167:203–217
Caro CG, Fitz-Gerald JM, Schroter RC (1971) Atheroma and arterial wall shear—observation, correlation, and proposal of a shear-dependent mass-transfer mechanism for atherogenesis. Proc Soc Lond B177:109–159
Dartsch PC, Hämmerle H, Betz E (1986) Orientation of cultured arterial smooth muscle cells growing on cyclically stretched substrates. Acta anat 125:108–113
Dartsch PC, Hämmerle H (1986) Orientation response of arterial smooth muscle cells to mechanical stimulation. Eur J Cell Biol 41:339–346
Dartsch PC (1987) Das Zellskelett von kultivierten Gefäßwandzellen. Mikrokosmos 76:33–39
Davies PF, Remuzzi A, Gordon EJ, Dewey CF Jr, Grimbrone MA Jr (1986) Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci USA 83:2114–2117
Deck JD (1986) Endothelial cell orientation on aortic valves leaflets. Cardiovasc Res 20:760–767
DeForrest JM, Hollis TM (1978) Shear stress and aortic histamine synthesis. Am J Physiol:H701–H705
Dewey CF Jr, Bussolari SR, Gimbrone MA Jr, Davies PF (1981) The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Engineering 103:177–185
Dewey CF Jr, Gimbrone MA Jr, Bussolari SR, White GE, Davies PF (1983) Response of vascular endothelium to unsteady fluid shear stress in vitro. In: Schettler G, et al. (eds) Fluid dynamics as a localizing factor for atherosclerosis. Springer-Verlag, Heidelberg, pp 182–187
Dobrin PB (1978) Mechanical properties of arteries. Physiol Rev. 58:397–460
Drenckhahn D (1983) Cell motility and cytoplasmic filaments in vascular endothelium. Prog appl Microcirculation 1:53–70
Drenckhahn D, Gress T, Franke RP (1986) Vascular endothelial stress fibres: their potential role in protecting the vessel wall from rheological damage. Klin. Wochenschr 64:986–988
Eskin SG, Ives CL, McIntire LV, Navarro LT (1984) Response of cultured endothelial cells to steady flow. Microvasc Res 28:87–94
Eskin SG, Ives CL, Frangos JA, McIntire LV (1985) Cultured endothelium: the response to flow. ASAIO J 8:109–112
Flaherty JT, Pierce JE, Ferrans VJ, Patel DJ, Tucker WK, Fry DL (1972) Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ Res 30:23–33
Frangos JA, Eskin SG, McIntire LV, Ives CL (1985) Flow effects on prostacyclin production by cultured human endothelial cells. Science 227:1477–1479
Franke RP, Gräfe M, Schnittler H, Seiffge D, Mittermayer C (1984) Induction of human vascular endothelial stress fibres by fluid shear stress. Nature 307:648–649
Franke RP, Gräfe M, Dauer U, Schnittler H, Mittermayer C (1986) Stress fibres in human endothelial cells under shear stress. Klin Wochenschr 64:989–992
Franke RP, Höpken S, Schnittler HJ, Fuhrmann R, Dauer U, Zangs R, Hofstädter F, Mittermayer C (1987) Wirkung von Scherkräften auf humane Endothelzellen. In: Betz E (ed) Frühveränderungen bei der Atherogenese. W. Zuckschwerdt Verlag, München, pp 56–61
Fry DL (1968) Acute vascular endothelial changes associated with increased blood velocity gradients. Circ Res 22:165–197
Fry DL (1976) Hemodynamic forces in atherogenesis. In: Steingerg P (ed) Cerebrovascular diseases. Raven Press, pp 77–95
Gabbiani G, Gabbiani F, Lombardi D, Schwartz SM (1983) Organization of actin cytoskeleton in normal and regenerating arterial endothelial cells. Proc Natl Acad Sci USA 80:2361–2364
Glagov S (1972) Hemodynamic risk factors: mechanical stress, mural architecture, medial nutrition, and the vulnerability of arteries to atherosclerosis. In: Wissler RW, Geer JC (eds) The pathogenesis of atherosclerosis. Williams and Wilkins, Baltimore, pp 164–199
Gotlieb AI, McBurnie May L, Subrahmanyan L, Kalnins VI (1981) Distribution of microtubule organizing centers in migrating sheets of endothelial cells. J Cell Biol 91:589–594
Gundersen GG, Bulinski JC (1988) Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci USA 85:5946–5950
Ives CL, Eskin SG, McIntire LV, DeBakey ME (1983) The importance of cell origin and substrate in the kinetics of endothelial cell alignment in response to steady flow. Trans Am Soc Artif Intern Organs 29:269–274
Ives CL, Eskin SG, McIntire LV (1986) Mechanical effects on endothelial cell morphology: an in vitro assessment. In Vitro Cell Development Biol 22:500–507
Kalnins VI, Connolly, JA (1981) Application of immunofluorescence in studies of cytoskeletal antigens. Adv Cell Neurobiol 2:393–460
Krueger JW, Young DF, Cholvin NR (1971) An in vitro study of flow response by cells. J Biomechanics 4:31–36
Langille BL, Adamson SL (1981) Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice. Circ Res 48:481–488
Leung DYM, Glagov S, Mathews MB (1976) Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science 191:475–477
Leung DYM, Glagov S, Mathews MB (1977) A new in vitro system for studying cell response to mechanical stimulation. Exp Cell Res 109:285–298
Levesque MJ, Nerem RM (1985) The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Engineering 107:341–347
Levesque MJ, Liepsch D, Moravec S, Nerem RM (1986) Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta. Arteriosclerosis 6:220–229
Levesque MJ, Nerem RM (1988) The influence of shear stress on vascular endothelial cell structure and function. In: Biology of the arterial wall-Satellite Meeting Siena, CIC Edizioni Internazionali, Rome, pp 175–182
Nerem RM, Levesque MJ, Cornhill JF (1980) Arterial fluid mechanics and the endothelium. In: Nerem RM, Guyton JR (eds) Hemodynamics in the arterial wall. University of Houston Press, Houston, Texas, pp 19–23
Netuschil L (1981) Vitalfärbung von Plaque-Mikroorganismen mit Fluoresceindiacetat und Ethidiumbromid. Dtsch zahnärztl Z 38:914–917
Ohara PT, Buck RC (1979) Contact guidance in vitro. A light, transmission, and scanning electron microscopic study. Exp Cell Res:235–249
Osborn M, Weber K (1982) Immunofluorescence and immunocytochemical procedures with affinity purified antibodies: tubulin-containing structures. Meth Cell Biol 24:97–132
Reidy MA, Langille BL (1980) The effect of local blood flow patterns on endothelial cell morphooogy. Exp Mol Pathol 32:276–289
Remuzzi A, Dewey CF Jr, Davies PF, Gimbrone MA Jr (1984) Orientation of endothelial cells in shear fields in vitro. Biorheology 21:617–630
Rhodin JG (1980) Architecture of the vessel wall. In: Bohr DF, Somlyo AP, Sparks HV (eds) Handbook of physiology, section 2: The cardiovascular system, Vol II. Vascular smooth muscle. American Physiological Society, Bethesda, Maryland, pp 1–31
Rogers KA, Kalnins VI (1983) Comparison of the cytoskeleton in aortic endothelial cells in situ and in vitro. Lab Invest 49:650–654
Rogers KA, McKee NH, Kalnins VI (1985) Preferential orientation of centrioles toward the heart in endothelial cells of major blood vessels is reestablished after reversal of a segment. Proc Natl Acad Sci USA 82:3272–3276
Ross R, Glomset JA (1976) The pathogenesis of atherosclerosis. New Engl J Med 295:369–377
Ross R, Glomset JA (1976) The pathogenesis of atherosclerosis. New Engl J Med 295:420–425
Ross R (1986) The pathogenesis of atherosclerosis — an update. New Engl J Med 314:488–500
Rotman B, Papermaster BW (1966) Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci USA 55:134–141
Sato M, Levesque MJ, Nerem RM (1987) Micropipette aspiration of cultured bovine aortic endothelial cells exposed to shear stress. Arteriosclerosis 7:276–286
Schultze Jena BS (1939) Über die schraubenförmige Struktur der Arterienwand. Gegenbaurs Morph Jb 83:230–246
Sottiurai VS, Kollros P, Glagov S, Zarins CK, Mathews MB (1983) Morphologic alteration of cultured arterial smooth muscle cells by cyclic stretching. J Surg Res 35:490–497
Sprague EA, Steinbach BL, Nerem RM, Schwartz CJ (1987) Influence of a laminar steady-state fluid imposed wall shear stress on the binding, internalization, and degradation of low-density lipoproteins by cultured arterial endothelium. Lab Invest 76:648–656
Sprague EA, Steinbach BL, Logan SA, Nerem RM, Schwartz CJ (1988) Influence of shear stress on lipoprotein endocytosis. In: Biology of the arterial wall — Satellite Meeting Siena. CIC Edizioni Internazionali, Rome, pp 183–188
Staubesand J (1959) Anatomie der Blutgefäße. I. Funktionelle Morphologie der Arterien, Venen und arterio-venösen Anastomosen. In: Ratschow M (ed) Angiologie. Thieme Verlag, Stuttgart, pp 23–82
Vandenburgh H, Kaufman S (1979) In vitro model for stretch-induced hypertrophy of skeletal muscle. Science 203:265–268
White GE, Fujiwara K, Shefton EJ, Dewey CF Jr, Gimbrone MA Jr (1982) Fluid shear stress influences cell shape and cytoskeletal organization in cultured vascular endothelium. Fed Proc 41:321
Wong AJ, Pollard TD, Herman IM (1983) Actin filament stress fibers in vascular endothelial cells in vivo. Science 219:867–869
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dartsch, P.C., Betz, E. Response of cultured endothelial cells to mechanical stimulation. Basic Res Cardiol 84, 268–281 (1989). https://doi.org/10.1007/BF01907974
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01907974