Abstract
Purpose
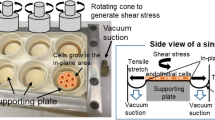
Vascular endothelial cells (ECs) are continuously subjected to mechanical forces such as fluid shear stress, stretching and hydrostatic pressure. The effect of hydrostatic pressure on EC responses has not been fully understood compared to that of the other two stimuli. The purpose of this study is to assess mechanical responses of ECs to these mechanical stimuli.
Methods
Bovine aortic ECs were exposed to hydrostatic pressure of 50, 100, and 150 mmHg and fluid shear stress of 3 Pa in simultaneous or successive fashion. Immunofluorescence staining of actin filaments and VEcadherin was then performed to observe cell morphology and cell-cell junctions, respectively
Results
The results showed that ECs subjected to 50, 100, and 150 mmHg for 24 h elongated without predominant orientation and exhibited multilayered structure, whereas simultaneous application of 50 and 100 mmHg and 3 Pa for 24 h induced marked elongation and orientation of ECs parallel to the direction of flow maintaining monolayer integrity. This monolayer integrity was lost in ECs subjected to 150 mmHg together with 3 Pa. A successive application of 100 mmHg for 24 h followed by 100 mmHg and 3 Pa for 24 h, indicated that the loss of monolayer integrity due to hydrostatic pressure could not be retrieved by the following simultaneous application.
Conclusions
It can be concluded that physiological shear stress of 3 Pa is dominant to physiological hydrostatic pressure up to 100 mmHg, importantly suggesting the relative contribution of physiological hydrostatic pressure and fluid shear stress to endothelial monolayer integrity.
Similar content being viewed by others
References
Levesque MJ, Nerem RM. The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng. 1985; 107(4):341–7.
Dewey CF, Bussolari SR, Gimbrone MA, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981; 103(3):177–85.
Sakamoto N, Saito N, Han X, Ohashi T, Sato M. Effect of spatial gradient in fluid shear stress on morphological changes in endothelial cells in response to flow. Biochem Biophys Res Commun. 2010; 395(2):264–9.
Sakamoto N, Segawa K, Kanzaki M, Ohashi T, Sato M. Role of p120-Catenin in the morphological changes of endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 2010; 398(3):426–32.
Takemasa T, Yamaguchi T, Yamamoto Y, Sugimoto K, Yamashita K. Oblique alignment of stress fibers in cells reduces the mechanical stress in cyclically deforming fields. Eur J Cell Biol. 1998; 77(2):91–9.
Wang JH, Goldschmidt-Clermont P, Yin FC. Contractility affects stress fiber remodeling and reorientation of endothelial cells subjected to cyclic mechanical stretching. Ann Biomed Eng. 2000; 28(10):1165–71.
Wang JH, Goldschmidt-Clermont P, Wille J, Yin FC. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J Biomech. 2001; 34(12):1563–72.
Ohashi T, Masuda M, Matsumoto T, Sato M. Nonuniform strain of substrate induces local development of stress fibers in endothelial cells under uniaxial cyclic stretching. Clin Hemorheol Microcirc. 2007; 37(1-2):37–46.
Acevedo AD, Bowser SS, Gerritsen ME, Bizios R. Morphological and proliferative responses of endothelial cells to hydrostatic pressure: role of fibroblast growth factor. J Cell Physiol. 1993; 157(3):603–14.
Salwen SA, Szarowski DH, Turner JN, Bizios R. Three–dimensional changes of the cytoskeleton of vascular endothelial cells exposed to sustained hydrostatic pressure. Med Biol Eng Comput. 1998; 36(4):520–7.
Sugaya Y, Sakamoto N, Ohashi T, Sato M. Elongation and random orientation of bovine endothelial cells in response to hydrostatic pressure: comparison with response to shear stress. JSME Int J Ser C. 2003; 46(4):1248–55.
Ohashi T, Sugaya Y, Sakamoto N, Sato M. Hydrostatic pressure influences morphology and expression of VE–cadherin of vascular endothelial cells. J Biomech. 2007; 40(11):2399–405.
Müller-Marschhausen K, Waschke J, Drenckhahn D. Physiological hydrostatic pressure protects endothelial monolayer integrity. Am J Physiol Cell Physiol. 2008; 294(1):C324–32.
Moore JE, Bürki E, Suciu A, Zhao S, Burnier M, Brunner HR, Meister JJ. A device for subjecting vascular endothelial cells to both fluid shear stress and circumferential cyclic stretch. Ann Biomed Eng. 1994; 22(4):416–22.
Zhao S, Suciu A, Ziegler T, Moore JE, Bürki E, Meister JJ, Brunner HR. Synergistic effects of fluid shear stress and cyclic circumferential stretch on vascular endothelial cell morphology and cytoskeleton. Arterioscler Thromb Vasc Biol. 1995; 15(10):1781–6.
Owatverot TB, Oswald SJ, Chen Y, Wille JJ, Yin FC. Effect of combined cyclic stretch and fluid shear stress on endothelial cell morphological responses. J Biomech Eng. 2005; 127(3):374–82.
Wojciak-Stothard B, Tsang LY, Paleolog E, Hall SM, Haworth SG. Rac1 and RhoA as regulators of endothelial phenotype and barrier function in hypoxia-induced neonatal pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006; 290(6):L1173–82.
Seebach J, Dieterich P, Luo F, Schillers H, Vestweber D, Oberleithner H, Galla HJ, Schnittler HJ. Endothelial barrier function under laminar fluid shear stress. Lab Invest. 2000; 80(12):1819–31.
Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, De Paola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol. 2002; 26(4):453–64.
Hordijk PL, Anthony E, Mul FP, Rientsma R, Oomen LC, Roos D. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J Cell Sci. 1999; 112:1915–23.
Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady–state effects of shear stress on endothelial cell adherens junctions. Circ Res. 1999; 85(6):504–14.
Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007; 100(2):158–73.
Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA. 1999; 96(17):9815–20.
Masuda M, Fujiwara K. Morphological response of single endothelial cells exposed to physiological levels of shear stress. Front Med Biol Eng. 1993; 5(2):79–87.
Vestweber D. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008; 28(2):223–232.
Seebach J, Donnert G, Kronstein R, Werth S, Wojciak-Stothard B, Falzarano D, Mrowietz C, Hell SW, Schnittler HJ. Regulation of endothelial barrier function during flow-induced conversion to an arterial phenotype. Cardiovasc Res. 2007; 75(3):596–607.
dela Paz NG, Walshe TE, Leach LL, Saint-Geniez M, D’Amore PA. Role of shear-stress-induced VEGF expression in endothelial cell survival. J Cell Sci. 2012; 125:831–43.
Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008; 121:2115–22.
Shin HY, Smith ML, Toy KJ, Williams PM, Bizios R, Gerritsen ME. VEGF-C mediates cyclic pressure-induced endothelial cell proliferation. Physiol Genomics. 2002; 11(3):245–51.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohashi, T., Sugaya, Y., Sakamoto, N. et al. Relative contribution of physiological hydrostatic pressure and fluid shear stress to endothelial monolayer integrity. Biomed. Eng. Lett. 6, 31–38 (2016). https://doi.org/10.1007/s13534-016-0210-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-016-0210-x