Summary
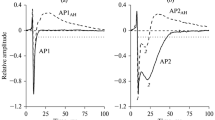
The effects of acute and chronic alloxan diabetes on the transmembrane electrical activity of rat heart papillary muscle were investigated. The action potential duration (APD) appeared markedly prolonged in all diabetic papillary muscles, as compared to normal. This prolongation of ADP, with no difference in the resting potential (RP), resulted from both a lengthening of the complex time course plateau and a slower rate of repolarisation. APD0 (at 0mV) and APD10 (+10mV from RP) increased, respectively, an average of 50% and 24% in the acute, and 72% and 98% in the chronic diabetics as compared to control, whereas Vmax and overshoot (OS) were unchanged. Varying [Ca l0 between 0.5 and 3.5 mM did not induce any change in the RP of either control or diabetic papillary muscles. Conversely, there were differences, within and between groups, in the amplitude of the OS and in Vmax, depending on the [Ca]o concentration. In particular, OS and Vmax of acute diabetics were markedly reduced at 1.5 mM. This reduction was maintained at concentrations of [Ca]o lower than 1.5, attesting to the greater sensitivity of both acutely and chronically diabetic muscles to a decrease in external calcium. Cd, a Ca-channel blocker, reduced in diabetics the duration of both the complex plateau and the repolarisation phase, suggesting that a Ca inward current was maintained throughout these two phases. Direct evidence for elucidating the mechanism(s) of the observed APD change in diabetics will be obtained only by transmembrane current analysis.
Similar content being viewed by others
References
Beeler GW, Reuter H (1970) Voltage clamp experiments on ventricular myocardial fibres. J Physiol 207:165–190
Bielefeld DR, Pace CS, Boshell BR (1983) Altered sensitivity of chronic diabetic rat heart to calcium. Am J Physiol 245:E560-E567
Brown AM, Morimoto K, Tsuda Y, Wilson DL (1981) Calcium current-dependent and voltagedepende inactivation of Ca channels. J Physiol 320:193–218
Chesnais JM, Sauviat MP, Vassas JM (1969) Effets de la substitution du lithium et du strontium au sodium et au calcium sur l'activité électrique cardiaque. J Physiol (Paris) 61:244
Cohen CJ, Bean BP, Tsien RW (1984) Maximal upstroke velocity as an index of available sodium conductance. Comparison of maximal upstroke velocity and voltage clamp measurements of sodium current in rabbit Purkinje fibers. Circ Res 54:636–651
Colquhoun D, Neher N, Reuter H, Stevens CF (1981) Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature 294:752–754
Coraboeuf E, Carmeliet E (1982) Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflügers Arch 392:352–359
Coraboeuf E, Vassort G (1968) Effects of some inhibitors of ionic permeabilities on ventricular action potential and contraction of rat and guinea-pig hearts. J Electrocardiol 1:19–30
Coraboeuf E (1982) Ionic basic of electrical activity in cardiac tissues. In: Levy MN, Vassalle M (eds) Excitation and neural control of the heart. American Physiological Society, Bethesda, 1–35
Fein FS, Kornstein IB, Strobeck JE, Capasso JM, Sonnenblick EH (1980) Altered myocardial mechanics in diabetic rats. Circ Res 47:922–933
Fein FS, Aronson RS, Nordin C, Miller-Green B, Sonnenblick EH (1983) Altered myocardial response to ouabain in diabetic rats: mechanics and electrophysiology. J Mol Cell Cardiol 15:769–784
Feuvray D, Idell-Wenger JA, Neely JR (1979) Effects of ischemia on rat myocardial function and metabolism in diabetes. Circ Res 44:322–329
Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS (1983) Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol 244:E528-E535
Garber DW, Neely JR (1983) Decreased myocardial function and myosin ATPase in hearts from diabetic rats. Am J Physiol 244:H586-H591
Garber DW, Neely JR (1983) Cardiac function and myosin ATPase in diabetic rats treated with insulin, T3, and T4. Am J Physiol 244:H592-H598
Josephson IR, Sanchez-Chapula J, Brown AM (1984a) A comparison of calcium currents in rat and guinea-pig single ventricular cells. Circ Res 54:144–156
Josephson IR, Sanchez-Chapula J, Brown AM (1984b) Early outward current in rat single ventricular cells. Circ Res 54:157–162
Kass RS, Sanguinetti MC (1984) Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage and calcium-mediated mechanisms. J Gen Physiol 84:705–726
Kostyuk PG, Krishtal OA (1977) Separation of sodium and calcium currents in the somatic membrane of molluse neurones. J Physiol 270:545–568
Leblond Y, Sauviat MP, Berton J, Feuvray D (1984) Is the slow calcium conductance of rat papillary muscle altered by experimental diabetes (Abstract). J Mol Cell Cardiol 16 (Suppl 3):3
Lee KS, Tsien RW (1982) Reversal of current through calcium channels in dialysed single heart cells. Nature 297:498–501
Lee KS, Tsien RW (1983) Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature 302:790–794
Lopaschuk GD, Tahiliani AG, Vadlamudi RVSV, Katz S, McNeill JH (1983) Cardiac sarcoplasmic function in insulin-or carnitine-treated diabetic rats. Am J Physiol 245:H969-H976
Mitchell MR, Powell T, Terrar DA, Twist VW (1984) The effects of ryanodine. EGTA and lowsodium on action potentials in rat and guinea-pig ventricular myocytes: evidence for two inward currents during he plateau. Br J Pharmacol 81:543–550
Mitchell MR, Powell T, Terrar DA, Twist VW (1984) Strontium, nifedipine and 4-aminopyridine modify the time course of the action potential in cells from rat ventricular muscle. Br J Phamacol 81:551–556
Nordin C, Gilat E, Aronson RS (1985) Delayed afterdepolarizations and triggered activity in ventricular muscle from rats with streptozotocin-induced diabetes. Circ Res 57:28–34
Penpargkul S, Schaible T, Yipintsoi T, Scheuer J (1980) The effect of diabetes on performance and metabolism of rat hearts. Circ Res 47:911–921
Penpargkul S, Fein F, Sonnenblick EH, Scheuer J (1981) Depressed cardiac sarcoplasmic reticular function from diabetic rats. J Mol Cell Cardiol 13:303–309
Regan TJ, Haider B, Lyons MM (1978) Altered ventricular function and metabolism in diabetes mellitus. In: Zoneraich S (ed) Diabetes and the heart. Thomas, Springfield, Ill. p 123
Schouten VJA, Ter Keurs HEDJ (1985) The slow repolarization phase of the action potential in rat heart. J Physiol 360:13–25
Senges JJ, Brachmann J, Pelzer D, Hasslacher C, Weihe E, Kubler W (1980) Altered cardiac automaticity and conduction in experimental diabetes mellitus. J Mol Cell Cardiol 12:1341–1351
Sheu SS, Fozzard HA (1982) Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol 80:325–351
Siegelbaum SA, Tsien RW (1980) Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol 299:485–506
Tricoche R, Besseau A (1964) Electrogénèse du myocarde ventriculaire chez le rat diabétique. Action de l'insuline. J. Physiol (Paris) 56:663–664
Vadlamudi RVSV, McNeill JH (1983) Effect of experimental diabetes on rat cardiac cAMP, phosphorylase and inotropy. Am J Physiol 244:H844-H851
Weidmann S (1955) Effects of calcium ions and local anesthetics on electrical properties of Purkinje fibres. J Physiol 129:568–582
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sauviat, M.P., Feuvray, D. Electrophysiological analysis of the sensitivity to calcium in ventricular muscle from alloxan diabetic rats. Basic Res Cardiol 81, 489–496 (1986). https://doi.org/10.1007/BF01907755
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01907755