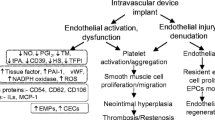
Abstract
The ubiquitous relationship of late graft occlusion, particularly the nonautogenous bypasses and distal anastomotic intimal hyperplasia (DAIH), has been extensively investigated. After having established the specific location of DAIH in human autogenous, biologic and prosthetic late graft occlusion, canine models were designed to elucidate the role of compliance mismatch between graft and the host artery and the configuration of the distal end to side versus end to end anastomosis using autogenous artery. DAIH occurred in all end to side anastomosis but not in side matching end to end anastomosis. Compliance mismatched is not the cause of DAIH development, but it enhances the pathogenesis. Further study using pharmacologic agents to modulate the activities of vascular myoblasts was successful in attenuating DAIH development and progression. Since DAIH occurs through a cascade of events initiated by endothelial lifting of the overlapping processes at the gap junction to allow blood borne elements to enter the subendothelial space, then incites myoblast proliferation and matrix synthesis leading to DAIH formation at the heel and toe where oscillatory flow exists. Control of myoblast activity is needed on a permanent basis to prevent DAIH development and progression. Cessation of treatment allows resurgence of DAIH biogenesis. Direct regulation of vascular myoblast activity preclude the need of inhibiting other source of stimuli that increases as our knowledge of the subject grows. Without the participation of myoblast, there will be no DAIH irrespective of the potency and numbers of enzymatic, hormonal, or mechanical inciting factors.
DAIH is a byproduct of oscillatory flow at the heel and the toe which enable blood borne elements to enter subendothelial space via the gap junction subsequent to lifting of endothelial cell overlapping processes. Pharmacologic control aiming at inactivating the myoblast in proliferation and matrix synthesis is a logical mode to control DAIH formation and late graft failure. Our experimental models confirm the success in controlling DAIH using heparin fraction that lack the anticoagulant yet is active in antiproliferative effect on myoblasts.
Similar content being viewed by others
References
Sottiurai VS, Yao JST, Flinn WR, Batson RC (1982) Intimal hyperplasia and neointima: An ultrastructural analysis of thrombosed grafts in humans. Surgery 93:809–817.
Sottiurai VS, Yao JST, Batson RC, Lim Sue S, Jones R, Nakamura YA (1989) Distal anastomotic intimal hyperplasia: Histopathologic character and biogenesis. Ann Vasc Surg 1:26–33.
Sottiurai VS (1989) The role of vein patch at distal anastomosis presented at 3rd European Society for Vascular Surgery. Malmo, Sweden. September 4, 1989.
Sottiurai VS, Lim Sue S, Feinberg EL II, Bringaze WL, Tran AT, Batson RC (1988) Distal anastomotic intimal hyperplasia: Biogenesis and etiology. Eur J Vasc Surg 2:245–256.
Sottiurai VS, Lim Sue S, Breaux JR, Smith LM (1989) Adaptability of endothelial orientation to blood flow dynamics: A morphologic analysis. Eur J Vasc Surg 3:145–151.
Sottiurai VS (1990) Biogenesis and etiology of distal anastomotic intimal hyperplasia. Int Angiol 9:59–69.
Sottiurai VS (1990) Distal Anastomotic intimal hyperplasia in bypass grafts. In: Symposium—The Prosthetic Substitution of Blood Vessels—Actual State and Future Development, Volmar JF, Kogel H (eds). Quintessenz-Verlag GmbH: Berlin, Germany, pp 117–127.
Abbott WM, Megerman J, Hasson JE, L'Italien G, Warnock DF (1987) Effect of compliance mismatch on vascular graft patency. J Vasc Surg 5:376–382.
Imparato AM, Bracco A, Kim GE, Zeff R (1972) Intimal and neointimal fibrous proliferation causing failure of arterial reconstructions. Surgery 72:1007–1017.
Fry DL (1968) Acute vascular endothelial changes associated with increased blood velocity gradients. Circulation 12:65–97.
Caro CG, Fitzgerald JM, Schroter RC (1971) Atheroma and arterial wall shear: Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond [Biol] 177:109–159.
Gustein WH, Schneck DJ, Marks JO (1968) In vitro studies of local blood flow disturbance in a region of separation. J Atheroscler Res 8:3812–388.
Scharfstein H, Gustein WH, Lewis L (1963) Changes of boundary layer flow in model systems: Implications for initiation of endothelial injury. Cir Res 13:580–584.
Faulkner SL, Fisher RD, Conkle DM et al. (1975) Effect of blood flow rate on subendothelial proliferation in venous autografts used as arterial substitutes. Circulation 51:163–172.
Gustein WH, Farell GA, Armellini C (1973) Blood flow disturbance and endothelial cell injury in preatherosclerotic swine. Lab Invest 29:134–149.
Rittgers SE, Karayannacos PE, Guy JE, et al. (1978) Velocity distribution and intimal proliferation in autologous vein grafts in dogs. Circ Res 42:792–801.
Berguer R, Higgins RF, Redd DJ (1980) Intimal hyperplasia: An experimental study. Arch Surg 115:332–335.
Callow AD (1987) Presential address: The microcosm of the arterial wall—a plea for research. J Vasc Surg 5:1–18.
Callow AD, Connolly R, O'Donnel Jr TE, et al. (1982) Plateletarterial synthetic graft interaction and its modification. Arch Surg 117:1447–1455.
Stanley JC, Burkel WE, Ford JW, et al. (1982) Enhanced patency of small diameter, externally supported Dacron iliofemoral grafts seeded with endothelial cells. Surgery 92:994–1005.
Herring M, Dilley R, Jersild RA Jr, et al. (1979) Seeding arterial prostheses with viscular endothelium. The nature of the lining. Ann Surg 190:84–90.
Roseman JE, Kempczinski RF, Pearce WH, et al. (1985) Kinetics of endothelial cell seeding. J Vasc Surg 2:778–784.
Loskutoff DJ, Mussoni L (1983) Interactions between fibrin and the plasminogen activators produced by cultured endothelial cells. Blood 62:62–68.
Sottiurai VS, Batson RC (1984) Ultrastructural studies of arterial grafts. In: Evaluation of Treatment of Upper and Lower Extremity Circulation Disorders, Bergan JJ, Yao JST (eds). Grune and Stratton: New York, pp 371–387.
Author information
Authors and Affiliations
About this article
Cite this article
Sottiurai, V.S. Distal anastomotic intimal hyperplasia: Histocytomorphology, pathophysiology, etiology, and prevention. International Journal of Angiology 8, 1–10 (1999). https://doi.org/10.1007/BF01616834
Issue Date:
DOI: https://doi.org/10.1007/BF01616834