Summary
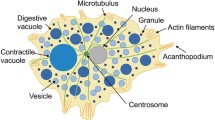
Behaviour of the membrane and contractile system was directly recorded in the advancing and retracting frontal zones of spontaneously locomoting or stimulated amoebae. The advancing pseudopodial tips alternately slow down and accelerate. In the slowing phase the frontal hyaline caps are flat and compressed by countercontraction of the cortical actin network beneath the leading edge. At this stage the membrane-cytoskeleton complex splits: the detached contractile layer is retracted inwards, and the membrane lifted outwards. The fluid endoplasm fraction is filtered forward through the detached actin network. This results in a local hydrostatic pressure drop, immediately restores the forward flow of endoplasm and initiates the acceleration phase of the leading edge progression. The frontal membrane, temporarily disconnected from the cytoskeletal layer, is free to slide and extend forward, but the new submembrane contractile network is soon repolymerized. In this way, after making one step forward, the frontal zone recovers its former state, and the cycle is then repeated. The cortex disassembly-reassembly cycles at the leading edge are produced every 2 s, on average. Retraction of the frontal contractile layers is part of the general centripetal cortex flow observed during motor functions of amoebae and many other cells, and is therefore associated with various other backward movements observed within and on the surface of advancing frontal zones of amoebae. The backward movement of the contractile cortex is also responsible for the withdrawal of previously advancing pseudopodia, if the detachment of successive contractile sheets from the frontal membrane ceases. It was demonstrated that the action of attractants and repellents is based on the activation or inhibition, respectively, of rhythmic disassembly of the membrane-cytoskeleton complex at the leading edge.
Similar content being viewed by others
References
Allen RD (1961) Ameboid movement. In: Brachet J, Mirsky AE (eds) The cell, vol2. Academic Press, New York, pp 135–216
Anderson OR (1988) Comparative protozoology. Springer, Berlin Heidelberg New York Tokyo, pp 366–374
Bereiter-Hahn J, Strohmeier R (1987 a) Biophysical aspects of motive force generation in tissue culture cells and protozoa. In: Wohlfarth-Bottermann KE (ed) Nature and function of cytoskeletal proteins in motility and transport. Fischer, Stuttgart, pp 1–15 (Fortschritte der Zoologie, vol 34)
— — (1987 b) Hydrostatic pressure in metazoan cells in culture: its involvement in locomotion and shape generation. In: Bereiter-Hahn J, Anderson OR, Reif WE (eds) Cytomechanics. Springer, Berlin Heidelberg New York Tokyo, pp 261–272
Bourguignon LYW, Bourguignon GJ (1984) Capping and the cytoskeleton. Int Rev Cytol 87: 195–224
Bray D, White JG (1988) Cortical flow in animal cells. Science 239: 883–888
Bretscher MS (1984) Endocytosis: relation to capping and cell location. Science 224: 681–686
Chen WT (1981) Mechanism of retraction of the trailing edge during fibroblast movement. J Cell Biol 90: 187–200
Couchman JR, Rees DA (1985) Coupling of cytoskeleton functions for fibroblast locomotion. Eur J Cell Biol 36: 182–194
Cramer EB, Gallin JI (1979) Localization of submembraneous cations to the leading end of human neutrophils during chemotaxis. J Cell Biol 82: 369–379
Czarska L, Grębecki A (1966) Membrane folding and plasma-membrane ratio in the movement and shape transformation inAmoeba proteus. Acta Protozool 4: 201–239
DeBiasio RL, Wang LL, Fisher GW, Taylor DL (1988) The dynamic distribution of fluorescent analogues of actin and myosin in protrusions of the leading edge of migrating Swiss 3 T 3 fibroblasts. J Cell Biol 197: 2631–2645
Dembo M (1989) Mechanics and control of the cytoskeleton inAmoeba proteus. Biophys J 55: 1053–1080
DiPasquale A (1975) Locomotory activity of epithelial cells in culture. Exp Cell Res 94: 191–215
Dołowy K (1987) Membrane potential-controlled lipid vesicle recycling and amoeboid locomotion. J Bioelectr 6: 109–128
Dunn GA (1980) Mechanisms of fibroblast locomotion. In: Curtis ASG, Pitts JD (eds) Cell adhesion and motility. Cambridge University Press, Cambridge, pp 409–423
Fisher WG, Conrad PA, DeBiasio RL, Taylor DL (1988) Centripetal transport of cytoplasm, actin, and the cell surface in lamellipodia of fibroblasts. Cell Motil Cytoskeleton 11: 235–247
Fukui Y, Yumura S (1986) Actomyosin dynamics in chemotactic amoeboid movement ofDictyostelium. Cell Motil Cytoskeleton 6: 662–673
Gawlitta W, Stockem W, Wehland J, Weber K (1980) Organization and spatial arrangement of fluorescein-labeled native actin microinjected into normal locomoting and experimentally influencedAmoeba proteus. Cell Tissue Res 206: 181–191
Grębecka L (1988) Polarity of the motor functions inAmoeba proteus. I. Locomotory behaviour. Acta Protozool 27: 83–96
—, Grębecki A (1981) Testing motor functions of the frontal zone in the locomotion ofAmoeba proteus. Cell Biol Int Rep 5: 587–594
—, Hrebenda B (1979) Topography of cortical layer inAmoeba proteus as related to the dynamic morphology of moving cell. Acta Protozol 18: 493–502
—, Grębecki A, Hrebenda B (1987) Motor behaviour ofAmoeba proetus during glutaraldehyde fixation. Acta Protozool 26: 31–38
Grębecki A (1980) Behaviour ofAmoeba proteus exposed to lightshade difference. Protistologica 16: 103–116
— (1981) Effects of localized photic stimulation on amoeboid movement and their theoretical implications. Eur J Cell Biol 24: 163–175
- (1982) Supramolecular aspects of amoeboid movement. In: Progress in protozoology. Proc VI Int Congr Protozool, part I, pp 117–130
— (1984) Relative motion inAmoeba proteus in respect to the adhesion sites. I. Behaviour of monotactic forms and the mechanism of fountain phenomenon. Protoplasma 123: 116–134
— (1985) Relative motion inAmoeba proteus in respect to the adhesion sites. II. Ectoplasmic and surface movements in polytactic and heterotactic amoebae. Protoplasma 127: 31–45
— (1986) Two-directional pattern of movements on the cell surface ofAmoeba proteus. J Cell Sci 83: 23–35
— (1987 a) Velocity distribution of the anterograde and retrograde transport of extracellular particles byAmoeba proteus. Protoplasma 141: 126–134
— (1987 b) Locomotion ofSaccamoeba limax. Arch Protistenk 134: 347–365
— (1988) Bidirectional transport of extracellular material by the cell surface of locomotingSaccamoeba limax. Arch Protistenk 136: 139–151
—, Kwiatkowska EM (1988) Dynamics of the membrane-cortex contacts demonstrated in vivo inAmoeba proteus pretreated by heat. Eur J. Protistol 23: 262–272
- Zaleska E (1986) Membrane-cortex interactions revealed in vivo in heat-pretreatedAmoeba proteus. In: II Eur Congr Cell Biol. Acta Biol Hung [Suppl] 37: Abstract 695
—, Grębecka L, Klopocka W (1981) Testing steering functions of the frontal zone in the locomotion ofAmoeba proteus. Cell Biol Int Rep 5: 595–600
Haberey M, Wohlfarth-Bottermann KE, Stockem W (1969) Pinocytose und Bewegung von Amöben. VI. Kinematographische Untersuchungen über das Bewegungsverhalten der Zelloberfläche vonAmoeba proteus. Cytobiologie 1: 70–84
Heath JP (1981) Arcs: curved microfilament bundles beneath the dorsal surface of the leading lamellae of moving chick embryo fibroblasts. Cell Biol Int Rep 5: 975–980
— (1983) Behaviour and structure of the leading lamella in moving fibroblasts. I. Occurrence and centripetal movement of arc-shaped microfilament bundles beneath the dorsal cell surface. J Cell Sci 60: 331–354
Hoffmann HU, Stockem W, Gruber B (1984) Dynamics of the cytoskeleton inAmoeba proteus. II. Influence of different agents on the spatial organization of microinjected fluorescein-labeled actin. Protoplasma 119: 79–92
Klopocka W, Stockem W, Grębecki A (1988) Fine structure and distribution of contractile layers inAmoeba proteus preincubated at high temperature. Protoplasma 147: 117–124
Kolega J (1985) Effects of mechanical tension on protrusive activity and microfilament and intermediate filament organization in an epidermal epithelium moving in culture. J Cell Biol 102: 1400–1412
Kolodziejczyk J, Grębecki A (1989) Dynamics of the submembrane contractile system in caffeine-derived protoplasmic droplets ofPhysarum polycephalum. Acta Protozool 28: 1–10
Kwiatkowska EM, Grębecki A (1988) Dissociation of membrane-cortex contacts in the hyalospheres ofAmoeba proteus exposed to light-shade differences. Cell Biol Int Rep 12: 849–855
— — (1990) Response of the hyalospheres ofAmoeba proteus to direct electric current. Acta Protozool 29: 155–161
Low PS, Lloyd DH, Stein TM, Rogers JA (1979) Calcium displacement by local anaesthetics. J Biol Chem 254: 4119–4125
Luby-Phelps K, Taylor DL (1988) Subcellular compartmentalization by local differentiation of cytoplasmic organization. Cell Motil Cytoskeleton 10: 28–37
Masayoshi K, Sato A (1978) Circular distribution of microfilaments in cell spreading in vitro. Exp Cell Res 113: 222–226
Mast SO (1926) Structure, movement, locomotion and stimulation in amoeba. J Morphol 41: 347–425
McRobbie SJ, Newell PC (1983) Changes in actin associated with the cytoskeleton following chemotactic stimulation ofDictyostelium discoideum. Biochem Biophys Res Commun 115: 351–359
Mittal AK, Bereiter-Hahn J (1985) Ionic control of locomotion and shape of epithelial cells. I. Role of calcium influx. Cell Motil 5: 123–136
Nachmias VT, Sullender JS, Fallon JR (1979) Effects of local anaesthetics on human platelets: filopodial suppression and endogenous proteolysis. Blood 53: 63–72
Onuma EK, Hui SW (1988) Electric field-directed cell shape changes, displacement and cytoskeletal reorganization are calcium dependent. J Cell Biol 106: 2067–2075
Oster GF (1984) On the crawling of cells. J Embriol Exp Morphol 83 [Suppl]: 329–364
Pagh KI, Adelman MR (1988) Assembly, disassembly, and movements of the microfilament-rich ridge during the amoeboflagellate transformation inPhysarum polycephalum. Cell Motil Cytoskeleton 11: 223–234
Puytorac P, Grain J, Mignot JP (1987) Précis de protistologie. Société Nouvelle des Éditions Boubée, Paris pp 221–223
Sanger JW, Mittal B, Sanger JM (1984) Interaction of fluorescentlylabeled contractile proteins with the cytoskeleton in cell models. J Cell Biol 99: 918–928
Sawyer DW, Sullivan JA, Mandell GL (1985) Intracellular free calcium localization in neutrophils during phagocytosis. Science 230: 663–666
Sheterline P, Gallagher K, Rickard JE, Richards RC (1984) Control of actin assembly in the cortical contractile network of neutrophil leukocytes during phagocytosis. J Submicrosc Cytol 16: 57–58
Singer SJ, Kupfer A (1986) The directed migration of eukaryotic cells. Ann Rev Cell Biol 2: 337–365
Soranngo T, Bell E (1982) Cytoskeletal dynamics of spreading and translocating cells. J Cell Biol 95: 127–136
Stockem W, Klopocka W (1988) Ameboid movement and related phenomena. Int Rev Cytol 112: 137–183
—, Hoffmann HU, Gawlitta W (1982) Spatial organization and fine structure of the cortical filament layer in normal locomotingAmoeba proteus. Cell Tissue Res 221: 505–519
— —, Gruber B (1983) Dynamics of the cytoskeleton inAmoeba proteus. I. Redistribution of microinjected fluorescein-labeled actin during locomotion, immobilization and phagocytosis. Cell Tissue Res 232: 79–96
—, Naib-Majani W, Wohlfarth-Bottermann KE, Osborn M, Weber K (1983) Pinocytosis and locomotion of amoebae. XIX. Immunocytochemical demonstration of actin and myosin inAmoeba proteus. Eur J Cell Biol 29: 171–178
Strohmeier R, Bereiter-Hahn J (1984) Control of cell shape and locomotion by external calcium. Exp Cell Res 154: 412–420
Taylor DL, Fechheimer M (1982) Cytoplasmic structure and contractility: the solation-contraction coupling hypothesis. Philos Trans R Soc Lond [Biol] 299: 185–197
—, Wang YL, Heiple J (1980 a) The contractile basis of ameboid movement. VII. The distribution of fluorescently labeled actin in living amoebas. J Cell Biol 86: 590–598
—, Blinks JR, Reynolds G (1980 b) The contractile basis of ameboid movement. VIII. Aequorin luminescence during ameboid movement, endocytosis and capping. J Cell Biol 86: 599–607
Truett AP, Verghese MW, Dillon SB, Snyderman R (1988) Calcium influx stimulates a second pathway for sustained diacylglycerol production in leukocytes activated by chemoattractants. Proc Natl Acad Sci USA 85: 1549–1553
Wang LL, Bryan J (1881) Isolation of calcium dependent platelet proteins that interact with actin. Cell 25: 637–649
Wehland J, Weber K, Gawlitta W, Stockem W (1979) Effects of the actin-binding protein DNase I on cytoplasmic streaming and ultrastructure ofAmoeba proteus. Cell Tissue Res 199: 353–372
Wuestehube LJ, Luna EJ (1987) F-actin binds to the cytoplasmic surface of ponticulin, a 17-kD integral glycoprotein fromDictyostelium discoideum plasma membranes. J Cell Biol 105: 1741–1751
Yin HL, Hartwig JH (1988) The structure of the macrophage actin skeleton. J Cell Sci [Suppl 9]: 169–184
—, Albrecht JH, Fattoum A (1981) Identification of gelsolin, aCa2+-dependent regulatory protein of actin gel-sol transformation, and its intracellular distribution in a variety of cells and tissues. J Cell Biol 91: 901–906
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grębecki, A. Dynamics of the contractile system in the pseudopodial tips of normally locomoting amoebae, demonstrated in vivo by video-enhancement. Protoplasma 154, 98–111 (1990). https://doi.org/10.1007/BF01539837
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01539837