Summary
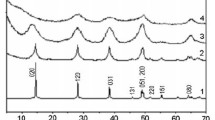
Gels and sols of aluminum oxide trihydrate were prepared by five different methods and studied by x-ray diffraction and electron microscopy. The x-ray diffraction analysis has shown that all methods produce mixtures of Gibbsite and Bayerite, the relative proportions of each varying from method to method. A sol and gel prepared by the same method present identical x-ray diffraction patterns and their morphologies are similar in the electron microscope. Two main particle shapes were observed: hexagonal prisms and triangular somatoids. There is experimental evidence which indicates that the former are Gibbsite and the latter, Bayerite. On this basis Willstaetter's methods seem to produce more Bayerite than Gibbsite. The reverse was true for Kraut's method, while in Fricke and Jockers' method, almost pure Bayerite was produced. A platelet structure is proposed for the triangular somatoids.
Similar content being viewed by others
Bibliography
Willstaetter, R., Untersuchungen über Enzyme, p. 141 et seq., (Berlin 1928).
Fricke, R. and Meyring, K., Z. anorg. allg. Chem.214, 269 (1933).
Milligan, W. O., J. Phys. Coll. Chem.55, 497 (1951).
Milligan, W. O. and J. L. McAtee, Problems of Clay and Laterite Genesis Symposium, p. 94, (1952).
5) Weiser, H. B., The Hydrous Oxides and Hydroxides, p. 2 and seq., (New York 1935).
Weiser, H. B. and W. O. Milligan, Advances in Colloid Science1, 227 (New York 1942).
Kraut, H., E. Flacke, W. Schmidt and H. Volmer, Ber.75, 1357 (1940).
Kohlschuetter, V., W. Beutler, L. Sprenger and M. Berlin, Helv. Chem. Acta14, 3, 305, 331 (1931).
Fricke, R. and K. Jockers, Z. Naturforschg.2, 244 (1947).
Glemser, O., FIAT Review of German Science24, 42 (Wiesbaden 1948).
Souza Santos, P., A. Vallejo-Freire and H. L. Souza Santos, Kolloid-Z.133, 101 (1953);135, 56 (1954).
Feitknecht, W. and H. Studer, Kolloid-Z.115, 13 (1949).
Eberspaecher, O., Z. wissensch. Mikrosk.60, 393 (1952).
Frary, F. C., End. Eng. Chem.38, 129 (1946).
Beu, K. E. and Claassen, H. H. Rev. Sci. Inst.19, 179 (1948).
Beu, K. E., Rev. Sci. Inst.22, 62 (1951).
Watson, J. H. L., J. App. Phys.19, 713 (1948).
Kohlschuetter, V., Trans. Faraday Soc.31, 1181 (1935).
Watson, J. H. L., unpublished research.
Weiser, H. B., Colloid Chemistry, p. 4 (New York 1939).
Desch, C. H., Chemistry of Solids, p. 77 (1934); Guinier, A., Imperfections in Nearly Perfect Crystals, p. 402 (New York 1952).
Bunn, C. W. and H. Emmet, Discuss. Faraday Soc.5, 119 (1949).
Wyckoff, R. W. G., The Structure of Crystals (New York 1935).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Watson, J.H.L., Parsons, J., Vallejo-Freire, A. et al. X-ray and electron microscope studies on aluminium oxide trihydrates. Kolloid-Zeitschrift 140, 102–112 (1955). https://doi.org/10.1007/BF01510493
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01510493