Abstract
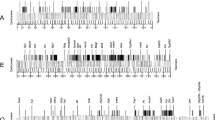
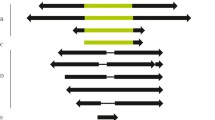
The distribution of the transposable elementBari-1 inD. melanogaster andD. simulans was examined by Southern blot analysis and byin situ hybridization in a large number of strains of different geographical origins and established at different times.Bari-1 copies mostly homogeneous in size and physical map are detected in all strains tested. Both inD. melanogaster and inD. simulans a relatively high level of intraspecific insertion site polymorphism is detectable, suggesting that in both speciesBari-1 is or has been actively transposing. The main difference between the two sibling species is the presence of a large tadem array of the element in a well-defined heterochromatic location of theD. melanogaster genome, whereas such a cluster is absent inD. simulans. The presence ofBari-1 elements with apparently identical physical maps in allD. melanogaster andD. simulans strains examined suggests thatBari-1 is not a recent introduction in the genome of themelanogaster complex. Structural analysis reveals unusual features that distinguish it from other inverted repeat transposons, whereas many aspects are similar to the widely distributedTc1 element ofC. elegans.
Similar content being viewed by others
References
Ashburner, M., 1989.Drosophila: A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
Anxolabéhère, D., M.G. Kidwell & G. Périquet, 1988. Molecular characteristics of diverse populations are consistent with a recent invasion ofDrosophila melanogaster by mobile P elements. Mol. Biol. Evol. 5:252–269.
Boussy, I.A. & S.B. Daniels, 1991. Hobo transposable elements inDrosophila melanogaster andD. simulans. Genet. Res. Camb. 58:27–34.
Brezinsky, L., G.V.L. Wang, T. Humphreys & J. Hunt, 1990. The transposable element Uhu from HawaiianDrosophila-member of the widely dispersed class of Tc1-like transposons. Nucleic Acids Res. 18:2053–59.
Caizzi, R., C. Caggese & S. Pimpinelli, 1993. Bari-1, a new transposon-like family inDrosophila melanogaster with a unique heterochromatic organization. Genetics 133:335–345.
Calvi, B.R., T.J. Hong, S.D. Findley & W.M. Gelbart, 1991. Evidence for a common evolutionary origin of inverted repeat transposon inDrosophila and plants: hobo, Activator, and Tam3. Cell 68:465–471.
Capy, P., D. Anxolabéhère & T. Langin, 1994. The strange phylogenies of transposable elements: are horizontal transfers the only explanation? Trends in Genetics 10:7–12.
Cariou, M. L., 1987. Biochemical phylogeny of the eight species in theDrosophila melanogaster species subgroup, includingD. sechellia andD. orena. Genet. Res. 50:181–185.
Cohn, V.H., M.A. Thompson & G.P. Moore, 1984. Nucleotide sequence comparison of the Adh gene in three drosophilids. J. Mol. Evol. 20:31–37.
Collins, J., E. Forbes & P. Anderson, 1939. The Tc3 family of transposable genetic elements inCaenorhabditis elegans. Genetics 121:47–55.
Crozatier, M., C. Vaury, I. Busseau, A. Pélisson & A. Bucheton, 1988. Structure and genomic organization of I elements involved in I-R hybrid dysgenesis inDrosophila melanogaster. Nucleic Acids Res. 16:9199–9213.
Daniels, S.B., A. Chovnick & I.A. Boussy, 1990. Distribution of hobo transposable elements in the genusDrosophila. Mol. Biol. Evol. 7:589–606.
Daniels, S.B., K.R. Peterson, L.D. Strausbaugh, M.G. Kidwell & A. Chovnick, 1990. Evidence for horizontal transmission of the P transposable element betweenDrosophila species. Genetics 124:339–355.
Danilevskaya, O.N., D.A. Petrov, M.N. Pavlova, A. Koga, E.V. Kurenova & D.L. Hartl, 1992. A repetitive DNA element, associated with telomeric sequences inDrosophila melanogaster, contains open reading frames. Chromosoma 102:32–40.
Di Nocera, P.P., F. Graziani & G. Lavorgna, 1986. Genomic and structural organization ofD. melanogaster G elements. Nucleic Acids Res. 14:675–691.
Emmons, S.W., L. Yesner, K.S. Ruan & D. Katzenberg, 1983. Evidence for a transposon inCaenorhabditis elegans. Cell 32:55–65.
Feinberg, A.P. & B. Vogelstein, 1983. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anl. Biochem. 132:6–13.
Finnegan, D.J. & D.H. Fawcett, 1986. Transposable elements inDrosophila melanogaster. In: N. Maclean (ed) Oxford surveys on eukaryotic genes, vol 3, pp 1–62.
Finnegan, D.J., 1989. Eukaryotic transposable elements and genome evolution. Trends in Genetics 5:103–107.
Franz, G. & C. Savakis, 1991. Minos, a new transposable element fromDrosophila hydei, is a member of the Tc1-like family of transposon. Nucleic Acids Res. 19:6646.
Ganetzky, B., 1977. On the component of segregation distortion inDrosophila melanogaster. Genetics 86:321–355.
Gerasimova, T., L. Mizrokhi & G. Georgiev, 1984. Transposition bursts in genetically unstableDrosophila melanogaster. Nature 309:714–716.
Heierhorst, J., K. Lederis & D. Richter, 1992. Presence of a member of the Tc1-like transposon family from nematode andDrosophila within the vasotocin gene of a primitive vertebrate, and the Pacific hagfishEptatretus stouti. Proc. Natl. Acad. Sci. USA 89: 6798–6802.
Henikoff, S., 1992. Detection ofCaenorhabditis transposon homologs in diverse organisms. New Biol. 4:382–388.
Henikoff, S. & R.H.A. Plasterk, 1988. Related transposon inC. elegans andD. melanogaster. Nucleic Acids Res. 16:6234.
Jacobson, J.W., M.M. Medhora & D.L. Hartl, 1986. Molecular structure of a somatically unstable transposable element inDrosophila. Proc. Natl. Acad. Sci. USA 83:8684–8688.
Jakubczak, J.L., Y. Xiong & T.H. Eickbush, 1990. Type I (R1) and Type II (R2) ribosomal DNA insertions ofDrosophila melanogaster are retrotransposable elements closely related to those ofBombyx mori. J. Mol. Biol. 212:37–52.
Karess, R.E. & G.M. Rubin, 1984. Analysis of P transposable element functions inDrosophila. Cell 38:135–146.
Kidwell, M.G., 1983. Evolution of hybrid dysgenesis determinants inDrosophila melanogaster. Proc. Natl. Acad. Sci. USA 80: 1655–1659.
Kidwell, M.G., 1992. Horizontal transfer. Curr. Opin. Genet. Develop. 2:868–873.
Kim, A., C. Terzian, P. Santamaria, A. Pelisson, N. Prudhomme & A. Bucheton, 1994. Retroviruses in Invertebrates — The Gypsy retrotransposon is apparently an infectious retrovirus ofDrosophila melanogaster. Proc. Nat. Acad. Sci. USA 91:1285–1289.
Lachaise, D., M.L. Cariou, J.R. David, F. Lemeunier, L. Tsacas & M. Ashburner, 1988. Historical biogeography of theDrosophila melanogaster species subgroup. Evol. Biol. 22:159–225.
Lankenau, D-H., P. Huijser, E. Jansen, K. Miedema & W. Hennig, 1990. DNA sequence comparison of micropia transposable elements fromDrosophila hydei andDrosophila melanogaster. Chromosoma 99:111–117.
Lemeunier, F., J.R. David, L. Tsacas & M. Ashburner, 1986. Themelanogaster species group, pp 147–256. The Genetics and Biology ofDrosophila, Vol 3e, edited by M. Ashburner & H.L. Carson, Academic Press, London.
MacDonald, J.F., 1993. Evolution and consequences of transposable elements. Curr. Opin. Genet. Develop. 3:855–864.
Maniatis, T., E.F. Fritsch & J. Sambrook, 1982. Molecular cloning: A Laboratory Manual. Cold Spring Harbor Laboratory. Cold Spring Harbor.
Maruyama, K. & D.L. Hartl, 1991a. Evidence for interspecific transfer of the transposable element mariner betweenDrosophila andZaprionus. J. Mol. Evol. 33:514–524.
Maruyama, K. & D.L. Hartl, 1991b. Evolution of the transposable element mariner inDrosophila species. Genetics 128:319–329.
Miklos, G.L.G., M.T. Yamamoto, J. Davies & V. Pirrotta, 1988. Microcloning reveals a high frequency of repetitive sequences characteristic of chromosome 4 and the beta-heterochromatin ofDrosophila melanogaster. Proc. Natl. Acad. Sci. USA 85: 2051–2055.
Mizrokhi, L.J. & A.M. Mazo, 1990. Evidence for horizontal transmission of mobile element jockey between distantDrosophila species. Proc. Natl. Acad. Sci. USA 87:9216–9220.
Mullins, M.C., D.C. Rio & G.M. Rubin, 1989. Cis-acting DNA sequence requirements for P-element transposition. Genes Dev. 3:729–738.
O'Hare, K. & G.M. Rubin, 1983. Structures of P transposable elements and their sites of insertion and excision in theDrosophila melanogaster genome. Cell 34:25–35.
Orgel, L.E. & F.H.C. Crick, 1980. Selfish DNA: the ultimate parasite. Nature 284:604–607.
Pardue, M.L., 1986. In situ hybridization to DNA of chromosomes and nuclei, pp. 11–137. InDrosophila: A Practical Approach, edited by D.B. Roberts, IRL Press, Oxford.
Pimpinelli, S. & P. Dimitri, 1989. Cytogenetic analysis of segregation distortion inDrosophila melanogaster: the cytological organization of the Responder (Rsp) locus. Genetics 121:765–772.
Robertson, H.M., 1993. The mariner transposable element is widespread in insects. Nature 362:241–245.
Rosenzweig, B., L.W. Liao & D. Hirsh, 1983. Sequence of theC. elegans transposable element Tc1. Nucleic Acids Res. 11: 4201–4209.
Rubin, G.M. & A.C. Spradling, 1982. Genetic transformation ofDrosophila with transposable element vectors. Science 218: 348–353.
Simmons, G.M., 1992. Horizontal transfer of hobo transposable elements within theDrosophila melanogaster species complex: Evidence from DNA sequencing. Mol. Biol. Evol. 9:1050–1060.
Simonelig, M., C. Bazin, A. Pelisson & A. Bucheton, 1988. transposable and nontransposable elements similar to the I factor involved in inducer-reactive (IR) hybrid dysgenesis inDrosophila melanogaster coexist in variousDrosophila species. Proc. Natl. Acad. Sci. USA 85:1141–1145.
Spradling, A.C. & G.M. Rubin, 1981.Drosophila genome organization: conserved and dynamic aspects. Annu. Rev. Genet. 15: 219–264.
Stacy, S.N., R.A. Lansman, H.W. Brock & T. Grigliatti, 1986. Distribution and conservation of mobile elements in the genusDrosophila. Mol. Biol. Evol. 6:522–534.
Streck, R.D., J.E. MacGaffey & S.K. Beckendorf, 1986. The structure of hobo transposable elements and their insertion sites. EMBO J. 5:3615–3623.
Throckmorton, L.H., 1975. The phylogeny, ecology, and geography ofDrosophila, pp 421–469. In Handbook of Genetics, edited by R.C. King, Plenum Press, New York.
Vaury, C., A. Bucheton & A. Pelisson, 1989. The β heterochromatic sequences flanking the I elements are themselves defective transposable elements. Chromosoma 98:215–224.
Wu, C.I., T.W. Lyttle, M.L. Wu & G.F. Lin, 1988. Association between a satellite DNA sequence and the Responder (Rsp) of Segregation Distorter inDrosophila melanogaster. Cell 54: 179–189.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Caggese, C., Pimpinelli, S., Barsanti, P. et al. The distribution of the transposable elementBari-1 in theDrosophila melanogaster andDrosophila simulans genomes. Genetica 96, 269–283 (1995). https://doi.org/10.1007/BF01439581
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01439581