Abstract
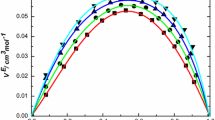
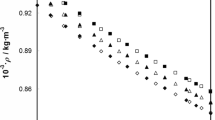
Excess volumes and isentropic compressibilities of 15 binary liquid mixtures containing methyl methacrylate (MMA). ethyl methacrylate (EMA), and butyl methacrylate (BM) andn-Hexane,n-heptane, carbon tetrachloride chlorobenzene ando-dichlorobenzene are derived from the measured densities and speeds of sound at 303.15 K. The dependence of the excess volumes and the isentropic compressibilities both on the alkyl chain length and on the nature of the solvent shots the dominance of dispersing interactions in the mixtures of aliphatic hydrocarbons and specific interactions im the chlorinated solvent mixtures. The speeds of sound of binary mixtures of MMA were found to be reasonably predicted by free length and collision factor theories. An attempt is also made to estimate the individual contributions of interactional. free volume andP * effects to the overall excess volumes of binary mixtures containing MMA. The results indicate that the three factors are equally responsible for the observed values.
Similar content being viewed by others
References
J. P. F. Grolier, D. Ballet and A. Viallard,J. Chem. Thermodyn. 6:895 (1974).
O. Dusart, J. P. E. Drolier, and A. Villard,Bull. Soc. Chim. Fr. 7:587 (1977).
L. Jimenez, L. Romani, M. I. Paz Andrade, G. Roux-Desgranges, and J. P. L. Grolier,J. Soln. Chem. 15: 379 (1986).
J. Ortega,Ber. Bunsenges. Phys. Chem. 92:1146 (1988).
J. Fernandez, L. Pias, J. Ortega and M. I. Paz Andrade,J. Chem. Thermodyn. 22:263 (1990).
J. Ortega,J. Chem. Thermodyn 23:327 (1991).
J. Ortega, E. Gonzalez, J.S. Matos and J.L. LegidoJ. Chem. Thermodyn. 24:15 (1992).
J. Ortega,J. Chem. Thermodyn. 24:1121 (1992).
J. Ortega and E. Gonzalez,J. Chem. Thermodyn. 25:495 (1993).
E. Gonzalez, J. Ortega, J. S. Matos, and G. Tardajos,J. Chem. Thermodyn. 25:561 (1993):25:801 (1993):26:41 (1994).
S. L. Oswal and I.N. Patel,Indian J. Chem. 29A:870 (1990).
B. Luo, S. E. M. Hamam, G.C. Benson and B.C.-Y. Lu,J. Chem. Thermodyn. 18:10143 (1986).
B. Luo, S. E. M. Hamam, G. C. Benson and B. C.-Y. LuJ. Chem. Eng. Data 32:81 (1937).
N. V. Sastry and M. M. Raj,Thermochim. Acta 257:39 (1995).
N. V. Sastry and M. M. Raj,Indian J. Chem. 35A:49 (1996).
J. H. Reddick and W. B. Bunger,“Organic Solvents” physical Properties and Methods of Purifications, 3d ed. (Wiley, New York 1970).
H. T. Van and D. Patterson,J. Soln. Chem. 11:793 (1982).
T. M. Letcher and R. C. Halter,J. Soln. Chem. 18:65 (1939).
A. Abe and P. J. Flory,J. Am. Chem. Soc. 87:1338 (1965).
M. T. Ratzsch, E. Richelt and H. Rosner,Z. Phys. Chem. Leipzig 255:933 (1974).
S. L. Oswal, B. M. Patel, H. R. Shah and P. Oswal,Int. J. Thermophys. 15:627 (1994).
J. A. Riddick, W. B. Hunger and T. K. Sakano,Organic Solvents Techniques of Chemistry Vol. 11 (Wiley, New York 1936).
J. Ortega and J. S. Matos,Mater. Chem. Phys. 15:415 (1986).
V.A. Bloomfield and R. K. Dewan,J. Phys. Chem. 75:31 (1975).
J. Zielkiewicz,J. Chem. Thermodyn. 26:959 (1994).
S.S. Joshi, T. Aminabhavi and S.S. Shukla,J. Chem. Eng. Data 35:247 (1990).
J. Nath and A. D. Triphati,J. Chem. Eng. Data 28:263 (1983).
K. S. Reddy,J. Chem. Eng. Data 31:238 (1986).
J. R. Sekar, P. R. Naidu, and W. E. Acree, Jr.,J. Chem. Eng. Data 38:167 (1993).
V.C. Kumar, B. Sreenivasulu and P. R. Naidu,J. Chem. Eng. Data 37:71 (1992).
J. P. Malia,Polymer 3:317 (1962).
J. F. Messerly, G. B. Guthrie, S. S. Todd and H. L. Finke,J. Chem. Eng. Data 12:338 (1967).
H. Kalai, F. Kholer, and P. Svejda,J. Chem. Eng. Data 37:133 (1992).
B. Jacobson,Acta. Chem. Scand. A,8:1485 (1952).
R. Nutsch-KunkesAcoustica 15:383 (1965).
W. Schaffs,Molekularakustich (Springer Verlag, Berlin, 1963), Chaps XI and XII.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sastry, N.V., Dave, P.N. Thermodynamics of acrylic esters containing binary liquid mixtures. I. Excess volumes and isentropic compressibilities of alkyl methacrylates +n-hexane, +n-heptane, + carbon tetrachloride, + chlorobenzene, ando-dichlorobenzene at 303.15 K. Int J Thermophys 17, 1289–1304 (1996). https://doi.org/10.1007/BF01438671
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01438671