Abstract
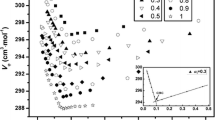
Micelle size and shape of dodecyldimethylammonium bromide have been determined by measurement of light scattering from its aqueous NaBr solutions. In water and in the presence of NaBr up to 0.07 M, the Debye plots give straight lines with positive slopes, and spherical micelles having molecular weight less than 30 000 are formed. At higher NaBr concentrations, the Debye plots decrease with increasing micelle concentration, indicating the aggregation of the primary spherical micelles into larger secondary micelles. The molecular weight and the radius of gyration of the secondary micelles increase with increasing NaBr concentration, and the relation between molecular weight and radius of gyration suggests that they are rodlike and flexible. Linear logarithmic relations between micelle molecular weight and ionic strength hold for spherical and rodlike micelles, respectively, and the threshold concentration of NaBr for the sphere-rod transition is located at 0.07 M. The spherical micelle of dodecyldimethylammonium ions has a size more than 20 surfactant ions larger in NaBr solutions than in NaCl solutions, and their rodlike micelle has a shorter length in NaBr solutions than in NaCl solutions, when compared at an identical aggregation number, indicating 2 more surfactant ions in its cross-section.
Similar content being viewed by others
References
Ikeda S, Ozeki S, Tsundoda M (1980) J Colloid Interface Sci 73:27
Hayashi S, Ikeda S (1980) J Phys Chem 84:744
Ikeda S, Hayashi S, Imae T (1981) J Phys Chem 85:106
Ozeki S, Ikeda S (1980) J Colloid Interface Sci 77:219
Ozeki S, Ikeda S (1981) Bull Chem Soc Jpn 54:522
Ozeki S, Ikeda S (1982) J Colloid Interface Sci 87:424
Anacker EW, Geer RD (1971) J Colloid Interface Sci 35:441
Geer RD, Eylar EH, Anacker EW (1971) J Phys Chem 75:369
Anacker EW, Ghose HM (1968) J Amer Chem Soc 90:3161
Elworthy PH Macfarlane CB (1963) J Chem Soc 907
Kushner LM, Hubbard WD (1955) J Colloid Sci 10:428
Mysels KJ, Princen LH (1959) J Phys Chem 63:1696
Anacker EW, Rush RM, Johnson JS (1964) J Phys Chem 68:81
Emerson M, Holtzer A (1967) J Phys Chem 71:1898
Huisman HF (1964) Proc Kon Ned Akad Wet, Ser B 67:388
Williams RJ, Phillips JN, Mysels KJ (1955) Trans Faraday Soc 51:728
Prins W, Hermans JJ (1956) Proc Kon Ned Akad Wet, Ser B 59:162
Princen LH, Mysels KJ (1957) J Colloid Sci 12:594
Vrij A, Overbeek JTG (1962) J Colloid Sci 17:570
Anacker EW, Ghose HM (1963) J Phys Chem 67:1713
Ford WPJ, Ottewill RH, Parreira HC (1966)J Colloid Interface Sci 21:522
Ozeki S, Ikeda S (1980) Bull Chem Soc Jpn 53:1832
Phillips JN (1955) Trans Faraday Soc 51:561
Mukerjee (1967) Advan Colloid Interface Sci 1:241
Tanford C (1979) The Hydrophobic Effect, 2nd Ed, Chap 6 and 7, John Wiley & Sons, New York
Anacker EW (ed) (1979) Mittal KL, Solution Chemistry of Surfactant, Vol 1, p 247, Plenum, New York & London
Barry BW, Russell GFJ (1972) J Colloid Interface Sci 40:174
Trap HJL, Hermans JJ (1955) Proc Kon Ned Akad Wet, Ser B 58:97
Tartar HV (1955) J Phys Chem 59:1195
Tartar HV (1959) J Colloid Sci 14:115
Tanford C (1972) J Phys Chem 76:3020
Israelachivili JN, Mitchell DJ, Ninham BW (1976) J Chem Soc Faraday Trans, II 72:1525
Leibner JE, Jacobus J (1977) J Phys Chem 81:130
Robson RJ, Dennis EA (1977) J Phys Chem 81:1075
Herrmann KW (1964) J Phys Chem 68:154
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ozeki, S., Ikeda, S. The sphere-rod transition of micelles of dodecyldimethylammonium bromide in aqueous NaBr solutions, and the effects of counterion binding on the micelle size, shape and structure. Colloid & Polymer Sci 262, 409–417 (1984). https://doi.org/10.1007/BF01410261
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01410261