Summary
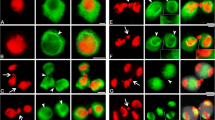
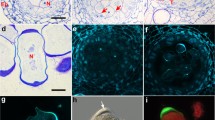
Changes in the microtubular cytoskeleton during meiosis and cytokinesis in hybrid moth orchids were studied by indirect immunofluorescence. Lagging chromosomes not incorporated into telophase nuclei after first meiotic division behave as small extra nuclei. Events in the microtubular cycle associated with these micronuclei are similar to and synchronous with those of the principal nuclei. During second meiotic division the micronuclei trigger formation of minispindles which are variously oriented with respect to the two principal spindles. After meiosis, radial systems of microtubules measure cytoplasmic domains around each nucleus in the coenocyte. Cleavage planes are established in regions where opposing radial arrays interact and the cytoplasm cleaved around micronuclei is proportionately smaller than that around the four principal nuclei. These observations clearly demonstrate that nuclei in plant cells are of fundamental importance in microtubule organization and provide strong evidence in support of our recently advanced hypothesis that division planes in simultaneous cytokinesis following meiosis are determined by establishment of cytoplasmic domains via radial systems of nuclear-based microtubules rather than by division sites established before nuclear division.
Similar content being viewed by others
Abbreviations
- DMSO:
-
dimethylsulfoxide
- FITC:
-
fluorescein isothiocyanate
- MTOC:
-
microtubule organizing center
- PBS:
-
phosphate buffered saline
- PPB:
-
preprophase band of microtubules
References
Bajer AS, Molè-Bajer J (1986) Reorganization of microtubules in endosperm cells and cell fragments of the higher plantHaemanthus in vivo. J Cell Biol 102: 263–281
Brown RC, Lemmon BE (1982) Ultrastructure of meiosis in the mossRhynchostegium serrulatum I. Prophasic microtubules and spindle dynamics. Protoplasma 110: 23–33
— (1987 a) Division polarity, development and configuration of microtubule arrays in bryophyte meiosis. I. Meiotic prophase to metaphase I. Protoplasma 137: 84–99
— (1987 b) Division polarity, development and configuration of microtubule arrays in bryophyte meiosis II. Anaphase I to the tetrad. Protoplasma 138: 1–10
— (1988 a) Sporogenesis in bryophytes. Adv Bryol 3: 159–223
— (1988 b) Microtubules associated with simultaneous cleavage in coenocytic microsporocytes. Am J Bot 75: 1848–1856
— (1988 c) Cytokinesis occurs in boundaries of cytoplasmic domains delimited by nuclear-based microtubules in sporocytes ofConocephalum conicum (Bryophyta). Cytoskel Cell Motil 11: 139–146
— (1988 d) Preprophasic microtubule systems and development of the mitotic spindle in hornworts. Protoplasma 143: 11–21
Busby CH (1986) Development of the meiotic cytoskeleton in bryophytes. MS Thesis, The Australian National University, Canberra
Church K, Nicklas RB, Lin H-P P (1986) Micromanipulated bivalents can trigger mini-spindle formation inDrosophila melanogaster spermatocyte cytoplasm. J Cell Biol 103: 2765–2773
Clayton L, Lloyd CW (1984) The relationship between division plane and spindle geometry inAllium cells treated with CIPC and griseofulvin: an anti-tubulin study. Eur J Cell Biol 34: 248–253
—, Black CM, Lloyd CW (1985) Microtubule nucleating sites in higher plant cells identified by an auto-antibody against peri-centriolar material. J Cell Biol 101: 319–324
Davis GL (1966) Systematic embryology of the angiosperms. Wiley, New York
De Mey J, Lambert A-M, Bajer AS, Moeremans M, De Brabander M (1982) The visualization of microtubules in interphase and mitotic plant cells ofHaemanthus endosperm with immuno-gold staining methods. Proc Natl Acad Sci USA 79: 1898–1902
Dover GA (1972) The organization and polarity of pollen mother cells ofTriticum aestivum. J Cell Sci 11: 699–711
Galatis B, Apostalakos P, Katsaros Chr (1983) Synchronous organization of two preprophase microtubule bands and final cell plate arrangement in subsidiary cell mother cells of someTriticum species. Protoplasma 117: 24–39
Gunning BES (1982) The cytokinetic apparatus: Its development and spatial regulation. In: Lloyd CW (ed) Cytoskeleton in plant growth and development. Academic Press, London New York, pp 229–292
Heslop-Harrison J (1971) Wall pattern formation in angiosperm microsporogenesis. Symp Soc Exp Biol 25: 277–300
Hogan CJ (1987) Microtubule patterns during meiosis in two higher plant species. Protoplasma 138: 126–136
Kubiac J, De Brabander M, De Mey J, Tarkowska JA (1986) Origin of the mitotic spindle in onion root cells. Protoplasma 130: 51–56
Lambert A-M (1980) The role of chromosomes in anaphase trigger and nuclear envelope activity in spindle formation. Chromosoma 76: 295–308
Marchant HJ, Pickett-Heaps JD (1973) Mitosis and cytokinesis inColeochaete scutata. J Phycol 9: 461–471
Molè-Bajer J, Bajer AS (1968) Studies of selected endosperm cells with the light and electron microscope. The technique. Cellule 67: 257–265+5 plates
Nicklas RB, Gordon GW (1985) The total length of spindle microtubules depends on the number of chromosomes present. J Cell Biol 100: 1–7
Pickett-Heaps JD, Northcote DH (1966) Organization of microtubules and endoplasmic reticulum during mitosis and cytokinesis in wheat meristems. J Cell Sci 1: 109–120
Schliwa M, van Berkom J (1981) Structural interaction of cytoskeletal components. J Cell Biol 90: 222–235
Schmit AC, Vantard M, De Mey J, Lambert A-M (1983) Aster-like microtubule centers establish spindle polarity during interphasemitosis transition in higher plant cells. Plant Cell Rep 2: 285–288
Sheldon JM, Dickinson HG (1983) Determination of patterning in the pollen wall ofLilium henryi. J Cell Sci 63: 191–208
— — (1986) Pollen wall formation inLilium: the effect of chaotropic agents, and the organization of the microtubular cytoskeleton during pattern development. Planta 168: 11–23
Sood SK, Rao PRM (1986) Gametophytes, embryogeny and pericarp ofMicrostylis wallichii Lindl. (Orchidaceae). Bot Mag Tokyo 99: 351–359
Steffen W, Fuge H, Dietz R, Bastmeyer M, Muller G (1986) Asterfree spindle poles in insect spermatocytes: evidence for chromosome-induced spindle formation? J Cell Biol 102: 1679–1687
Swamy BGL (1949) Embryological studies in theOrchidaceae. I. Gametophytes. Am Midl Nat 41: 184–201
Tiwari SC, Wick SM, Williamson RE, Gunning BES (1984) Cytoskeleton and integration of cellular function in cells of higher plants. J Cell Biol 99: 63 s-69 s
Wacker I, Quader H, Schnepf E (1988) Influence of the herbicide Oryzalin on cytoskeleton and growth ofFunaria hygrometrica protonemata. Protoplasma 142: 55–67
Waterkeyn L (1962) Les parois microsporocytaires de nature callosique chezHelleborus etTradescantia. Cellule 62: 225–255+3 plates
Wick SM (1985 a) The higher plant mitotic apparatus: redistribution of microtubules, calmodulin and microtubule initiation material during its establishment. Cytobios 43: 285–294
— (1985 b) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. III. Transition between mitotic/cytokinetic and interphase microtubule arrays. Cell Biol Int Rep 9: 357–371
—, Duniec J (1983) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. I. Preprophase band development and concomitant appearance of nuclear envelope-associated tubulin. J Cell Biol 97: 235–243
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brown, R.C., Lemmon, B.E. Minispindles and cytoplasmic domains in microsporogenesis of orchids. Protoplasma 148, 26–32 (1989). https://doi.org/10.1007/BF01403988
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01403988