Abstract
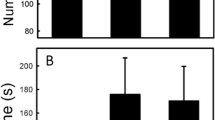
The ability to discriminate prey chemicals from control substances and the presence of a poststrike elevation in tongue-flicking (PETF) rate are experimentally demonstrated in the lacertid lizard,Podarcis muralis, The tongue-flick attack score, a composite index of response strength, was significantly higher in response to integumental chemicals from cricket than to cologne or distilled water. The cricket chemicals additionally elicited a significantly greater rate of tongue-flicking and higher proportion of attacks by the lizards than did control stimuli. PETF combined with apparent searching movements strongly suggest the presence of strike-induced chemosensory searching (SICS). Experimental evidence indicates that both PETF and SICS occur in insectivorous representatives of three families of actively foraging autarchoglossan lizards, suggesting their widespread occurrence in such lizards. The adaptive roles of chemosensory behavior in the foraging behavior of P.Muralis are discussed. It is proposed that these lizards may form chemical search images and that PETF and SICS may have been present in the lacertilian ancestors of snakes.
Similar content being viewed by others
References
Burghardt, G.M. 1970. Chemical perception in reptiles, pp. 241–308,in J.W. Johnston, D.G. Moulton, and A. Turk (eds.). Advances in Chemoreception, Vol. I. Communication by Chemical Signals. Appleton-Century-Crofts, New York.
Burghardt, G.M. 1973. Chemical release of prey attack: Extension to naive newly hatched lizards,Eumeces fasciatus.Copeia 1973:178–181.
Chiszar, D., andScudder, K.M. 1980. Chemosensory searching by rattlesnakes during predatory episodes, pp. 125–139,in D. Muller-Schwarze and R.M. Silverstein (eds.). Chemical Signals: Vertebrates and Aquatic Invertebrates. Plenum Press, New York.
Chiszar, D., Radcliffe, C.W., O'Connell, B., andSmith, H.M. 1982. Analysis of the behavioral sequence emitted by rattlesnakes during predatory episodes. II. Duration of strike-induced chemosensory searching in rattlesnakes (Crotalus viridis, C. enyo).Behav. Neural Biol. 34:261–270.
Chiszar, D. Radcliffe, C.W., Overstreet, R., Poole, andByers, T. 1985. Duration of strike-induced chemosensory searching in cottonmouthsAgkistrodon piscivorus and a test of the hypothesis that striking prey creates a specific search image.Can J. Zool. 63:1057–1061.
Cooper, W.E., Jr. 1989a. Absence of prey odor discrimination by iguanid and agamid lizards in applicator tests.Copeia 1989:472–478.
Cooper, W.E., Jr. 1989b. Prey odor discrimination in the varanoid lizardsHeloderma suspectum andVaranus exanthematicus.Ethology 81:250–258.
Cooper, W.E., Jr. 1989c. Strike-induced chemosensory searching occurs in lizards.J. Chem. Ecol. 15:1311–1320.
Cooper, W.E., Jr. 1990a. Prey odor detection by teiid and lacertid lizards and the relationship of prey odor detection to foraging mode in lizard families.Copeia 1990:237–242.
Cooper, W.E., Jr. 1990b. Prey odour discrimination by lizards and snakes, pp. 533–538,in D.W. Macdonald, D. Müller-Schwarze, and S.E. Natynczuk (eds.). Chemical Signals in Vertebrates 5. Oxford University Press, Oxford. In press.
Cooper, W.E., Jr. 1990c. Prey odor discrimination by anguid lizards.Herpetologica 46:183–190.
Cooper, W.E.,Jr. Prey odor discrimination and poststrike elevation in tongue-flicking by a cordylid lizard,Gerrhosaurus nigrolineatus. In press.
Cooper, W.E., Jr., andAlberts, A.C. 1990. Responses to chemical food stimuli by an herbivorous actively foraging lizard,Dipsosaurus dorsalis.Herpetologica 46:259–266.
Cooper, W.E., Jr., andAlberts, A.C. 1991. Tongue-flicking and biting in response to chemical food stimuli by an iguanid lizard (Dipsosaurus dorsalis) having sealed vomeronasal ducts: Vomerolfaction may mediate these behavioral responses.J. Chem. Ecol. 17:135–146.
Cooper, W.E., Jr., andBurghardt, G.M. 1990a. A comparative analysis of scoring methods for chemical discrimination of prey by squamate reptiles.J. Chem. Ecol. 16:45–65.
Cooper, W.E., Jr., andBurghardt, G.M. 1990b. Vomerolfaction and vomodor.J. Chem. Ecol. 16:103–105.
Cooper, W.E., Jr., andVitt, L.J. 1986. Thermal dependence of tongue-flicking and comments on use of tongue-flicking as an index of squamate behavior.Ethology 71:177–186.
Cooper, W.E., Jr., andVitt, L.J. 1989. Prey odor discrimination by the broad-headed skink (Eumeces laticeps).J. Exp. Zool. 249:11–16.
Cooper, W.E., Jr., McDowell, S.G., andRuffer, J. 1989. Strike-induced chemosensory searching in the colubrid snakesElaphe g. guttata andThamnophis sirtalis.Ethology 81:19–28.
Dial, B.E. 1978. Aspects of the behavioral ecology of two Chihuahuan desert geckos (Reptilia, Lacertilia, Gekkonidae).J. Herpetol. 12:209–216.
Dial, B.E., Weldon, P.J., andCurtis, B. 1989. Predator-prey signals: Chemosensory identification of snake predators by gekkonid lizards and its ecological consequences.J. Herpetol. 23:224–229.
Estes, R., Queiroz, K. De, andGauthier, J. 1988. Phylogenetic relationships within Squamata, pp. 119–281,in R. Estes and G. Pregill (eds.). Phylogenetic Relationships of the Lizard Families, Essays Commemorating Charles L. Camp. Stanford University Press, Stanford, California.
Frost, D.R., andEtheridge, R. 1989. A phylogenetic analysis and taxonomy of iguanian lizards (Reptilia: Squamata).Misc. Publ. Univ. Kans. Mus. Nat. Hist. 81:1–65.
Graves, B.M., andHalpern, M. 1990. Vomeronasal organ chemoreception in tongue-flicking, exploratory and feeding behaviour of the lizard,Chalcides ocellatus.Anim. Behav. 39:692–698.
Halpern, M. 1991. Nasal chemical senses in reptiles: Structure and function, pp. xxx-xxx,in D. Crews and C. Gans (eds.). Biology of the Reptilia, Vol. 18, Physiology and Behavior. University of Chicago Press, Chicago. In press.
Halpern, M., andFrumin, N. 1979. Roles of the vomeronasal and olfactory systems in prey attack and feeding in adult garter snakes.Physiol. Behav. 22:1183–1189.
Halpern, M., andKubie, J.L. 1980. Chemical access to the vomeronasal organs of garter snakes.Physiol. Behav. 24:367–371.
Hollander, A., andWolfe, D.A. 1973. Nonparametric Statistical Methods. John Wiley & Sons, New York.
Huey, R.B., andPianka, E.R. 1981. Ecological consequences of foraging mode.Ecology 62:991–999.
Kahmann, H. 1939. Uber das Jakobsonische Organ der Echsen.Z. Vergl. Physiol. 26:669–695.
Krekorian, C.O. 1989. Field and laboratory observations on chemoreception in the desert iguana.J. Herpetol. 23:267–273.
Loop, M.S., andScoville, S.A. 1972. Response of newbornEumeces inexpectatus to prey-object extracts.Herpetologica 28:254–256.
Nicoletto, P.F. 1985. The roles of vision and the chemical senses in predatory behavior of the skink,Scincella lateralis.J. Herpetol. 19:487–491.
Pedersen, J.M. 1988. Laboratory observations on the function of tongue extrusion in the desert iguana (Dipsosaurus dorsalis).J. Comp. Psychol. 102:193–196.
Regal, P.J. 1978. Behavioral differences between reptiles and mammals: An analysis of activity and mental capabilities, pp. 183–202,in N. Greenberg and P.D. MacLean (eds.). Behavior and Neurobiology of Lizards. United States Department of Health, Education, and Welfare, Washington, D.C.
Siegel, S. 1956. Nonparametric Statistics for the Behavioral Sciences. McGraw-Hill, New York.
Simon, C.A. 1983. A Review of Lizard Chemoreception, pp. 119–133 and 443–447,in R.B. Huey, E.R. Pianka, and T.W. Schoener (eds.). Lizard Ecology: Studies of a Model Organism. Harvard University Press, Cambridge, Massachusetts.
Simon, C.A., Gravelle, K., Bissinger, B.E., Eiss, I., andRuibal, R. 1981. The role of chemoreception in the iguanid lizardSceloporus jarrovi.Anim. Behav. 29:46–54.
Van Damme, R., Bauwens, D., Vanderstighelen, D., andVerheyen, R.F. 1990. Responses of the lizardLacerta vivipara to predator chemical cues: The effects of temperature.Anim. Behav. 40:298–305.
Vitt, L.J., andPrice, H.J. 1982. Ecological and evolutionary determinants of relative clutch mass in lizards.Herpetologica 38:237–255.
Von Achen, P.L., andRakestraw, J.L. 1984. The Role of Chemoreception in the Prey Selection of Neonate Reptiles, pp. 163–172,in R.A. Seigel, L.E. Hunt, J.L. Knight, L. Malaret, and N.L. Zuschlag (eds.). Vertebrate Ecology and Systematics-A Tribute to Henry S. Fitch. Special Publication, University of Kansas Museum of Natural History, Lawrence, Kansas.
Winer, B.J. 1962. Statistical Principles in Experimental Design. McGraw-Hill, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cooper, W.E. Responses to prey chemicals by a lacertid lizard,Podarcis muralis: Prey chemical discrimination and poststrike elevation in tongue-flick rate. J Chem Ecol 17, 849–863 (1991). https://doi.org/10.1007/BF01395595
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01395595