Abstract
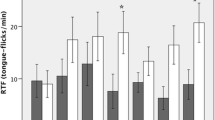
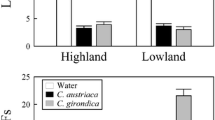
In a predator–prey interaction, the fitnesses of the predator and the prey depend on their abilities to recognize each other, a process that may involve different sensory modalities. Squamate reptiles are highly dependent on chemical senses for such recognition, and here we explored the ability of a generalist saurophagous snake, Philodryas chamissonis, to discriminate scents of two congeneric and sympatric lizard prey species, Liolaemus nitidus and L. chiliensis. A generalist saurophagous snake might just be sensitive to lizard scents in general, and if so, no discrimination between prey species is expected. However, these lizards use different substrates; L. nitidus basks on rocks, whereas L. chiliensis mainly basks on bushes and rarely on ground. The snake P. chamissonis basks on ground and rocks, and rarely on bushes. Therefore, if the rate of encounter affects the ability to recognize prey, we predict that P. chamissonis would show prey discrimination because scents of L. chiliensis may be encountered less frequently in its habitat. Results showed that the snake had a refined discrimination of lizard prey, reducing tongue flick rate and movements in response to scents from the common prey scents, L. nitidus. We also studied the ability of L. chiliensis to detect the snake and found that snake scents triggered a reduction in activity. The potential infrequent encounter between predator and prey may explain the asymmetric predator–prey recognition, as can be predicted from the “life-dinner” principle.


Similar content being viewed by others
References
Aguilar PM, Labra A, Niemeyer HM (2009) Self-chemical recognition in the lizard Liolaemus fitzgeraldi. J Ethol 27:181–184
Amo L, López P, Martín J (2004a) Chemosensory recognition of its lizard prey by the ambush smooth snake, Coronella austriaca. J Herpetol 38:451–454
Amo L, López P, Martín J (2004b) Thermal dependence of chemical assessment of predation risk affects the ability of wall lizards, Podarcis muralis, to avoid unsafe refuges. Physiol Behav 82:913–918
Arnold SJ (1992) Behavioural variation in natural populations. VI. Prey responses by two species of garter snakes in three regions of symapatry. Anim Behav 44:705–719
Balderas-Valdivia CJ, Ramírez-Bautista A (2005) Aversive behavior of beaded lizard, Heloderma horridum, to sympatric and allopatric predator snakes. Southwest Nat 50:24–31
Bevelander G, Smith TL, Kardong KV (2006) Microhabitat and prey odor selection in the foraging pigmy rattlesnake. Herpetologica 62:47–55
Bozinovic F, Rosenmann M (1988) Energetics and food requirements of the female snake Phillodryas chamissonis during the breeding season. Oecologia 75:282–284
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication. Sinauer Associates, China
Burghardt GM (1967) Chemical-cue preferences of inexperienced snakes: comparative aspects. Science 157:718–721
Cisterne A, Vanderduys EP, Pike DA, Schwarzkopf L (2014) Wary invaders and clever natives: sympatric house geckos show disparate responses to predator scent. Behav Ecol 25:604–611
Clark RW (2004a) Feeding experience modifies the assessment of ambush sites by the timber rattlesnake, a sit-and-wait predator. Ethology 110:471–483
Clark RW (2004b) Timber rattlesnakes (Crotalus horridus) use chemical cues to select ambush sites. J Chem Ecol 30:607–617
Cooper WE, Burghardt GM, Brown WS (2000) Behavioural responses by hatchling racers (Coluber constrictor) from two geographically distinct populations to chemical stimuli from potential prey and predators. Amphibia-Reptilia 21:103–115
Davies NB, Krebs JR, West SA (2012) An introduction to behavioural ecology. Wiley-Blackwell, Oxford
Dawkins R, Krebs JR (1979) Arms races between and within species. P Roy Soc B-Biol Sc 205:489–511
Dial BE, Schwenk K (1996) Olfaction and predator detection in Coleonyx brevis (Squamata: Eublepharidae), with comments on the functional significance of buccal pulsing in geckos. J Exp Zool 276:415–424
Downes SJ (2002) Does responsiveness to predator scents affect lizard survivorship? Behav Ecol Sociobiol 52:38–42
Downes SJ, Adams M (2001) Geographic variation in antisnake tactics: the evolution of scent-mediated behavior in a lizard. Evolution 55:605–615
Du WU, Webb JK, Shine R (2009) Heat, sight and scent: multiple cues influence foraging site selection by an ambush-foraging snake Hoplocephalus bungaroides (Elapidae). Curr Zool 55:266–271
Durand J, Legrand A, Tort M, Thiney A, Michniewicz RJ, Coulon A, Aubret F (2012) Effects of geographic isolation on anti-snakes responses in the wall lizard, Podarcis muralis. Amphibia-Reptilia 33:199–206
Escobar MAH, Vukasovic MA (2003) Predation of Philodryas chamissonis (Serpentes: Colubridae) on chicks of Aphrasthura [Aphrastura] spinicauda (Passeriformes: Furnariidae): an arboricolous snake? Not Men Mus Nac Hist Nat (Santiago) 352:18–20
Escobar CA, Labra A, Niemeyer HM (2001) Chemical composition of precloacal secretions of Liolaemus lizards. J Chem Ecol 27:1677–1690
Greenbaum E (2004) The influence of prey-scent stimuli on predatory behavior of the North American copperhead Agkistrodon contortrix (Serpentes: Viperidae). Behav Ecol 15:345–350
Greene HW, Jaksic FM (1992) The feeding behavior and natural history of two Chilean snakes, Philodryas chamissonis and Tachymenis chiliensis (Colubridae). Rev Chil Hist Nat 65:485–493
Hoare M, Labra A (2013) Searching for the audience of the weeping lizard’s distress call. Ethology 119:860–868
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Labra A (2008) Multi-contextual use of chemosignals by Liolaemus lizards. In: Hurst JL, Beynon RJ, Roberts SC, Wyatt TD (eds) Chemical signals in vertebrates 11. SpringerLink, New York, pp 357–365
Labra A (2011) Chemical stimuli and species recognition in Liolaemus lizards. J Zool 285:215–221
Labra A, Niemyer HM (1999) Intrspecific chemical recognition in the lizard Lioalemus tenuis. J Chem Ecol 25:1799–1811
Labra A, Niemeyer HM (2004) Variability in the assessment of snake predation risk by Liolaemus lizards. Ethology 110:649–662
Labra A, Pienaar J, Hansen TF (2009) Evolution of thermal physiology in Liolaemus lizards: adaptation, phylogenetic inertia, and niche tracking. Am Nat 174:204–220
Lobos G, Escobar MAH, Thomson RF, Alzamora A (2009) Philodryas chamissonis (long-tailed snake) and Liolaemus nitidus. Predation determined by pit tag. Herpetol Rev 40: 358
Mason RT, Parker MR (2010) Social behavior and pheromonal communication in reptiles. J Comp Physiol A 196:729–749
Mella JE (2005) Guía de campo Reptiles de Chile: Zona central. In Peñaloza AP, Novoa FF, Contreras M. Santiago, Chile, Centro de Ecología Aplicada Ltda, Pp xii + 147
Mori A, Hasegawa M (1999) Geographic difference in behavioral response of hatchling lizards (Eumeces okadae) to snake-predator chemicals. Jpn J Herpetol 18:45–56
Muñoz-Leal S, Ardiles K, Figueroa RA, González-Acuña D (2013) Philodryas chamissonis (Reptilia: Squamata: Colubridae) preys on the arboreal marsupial Dromiciops gliroides (Mammalia: Microbiotheria: Microbiotheriidae). Braz J Biol 73:15–17
Saviola AJ, Chiszar D, Mackessy SP (2012) Ontogenetic shift in response to prey-derived chemical cues in prairie rattlesnakes Crotalus viridis viridis. Curr Zool 58:549–555
Saviola AJ, Chiszar D, Smith HM, Mackessy SP (2013) Chemosensory response in stunted prairie rattlesnakes Crotalus viridis viridis. Curr Zool 59:175–179
Sepulveda M, Vidal MA, Farina JM (2006) Microlophus atacamensis (Atacama desert runner). Predation. Herpetol Rev 37:224–225
Shine R, Mason RT (2012) An airborne sex pheromone in snakes. Biol Lett 8:183–185
Telemeco RS, Baird TA, Shine R (2011) Tail waving in a lizard (Bassiana duperreyi) functions to deflect attacks rather than as a pursuit-deterrent signal. Anim Behav 82:369–375
Troncoso-Palacios J, Labra A (2012) Is the exploratory behavior of Liolaemus nitidus modulated by sex? Acta Herpetol 7:69–80
Van Damme R, Quick K (2001) Use of predator chemical cues by three species of lacertid lizards (Lacerta bedriagae, Podarcis tiliguerta, and Podarcis sicula). J Herpetol 35:27–36
Vidal MA, Labra A (2008) Herpetología de Chile. Science, Santiago, Chile
Weaver RE, Clark WH, McEwen DC (2012) Prey chemical discrimination by the desert Nightsnake (Hypsiglena chlorophaea): a comparison of invertebrate and vertebrate prey. J Herpetol 46:523–526
Webb JK, Du WG, Pike DA, Shine R (2009) Chemical cues from both dangerous and nondangerous snakes elicit antipredator behaviours from a nocturnal lizard. Anim Behav 77:1471–1478
Webb JK, Pike DA, Shine R (2010) Olfactory recognition of predators by nocturnal lizards: safety outweighs thermal benefits. Behav Ecol 21:72–77
Acknowledgments
The study was authorized by SAG (Resolution No.7266) and by the Scientific Ethics Committee of the Faculty of Medicine, University of Chile. We thank K. Aguilera, F. Contreras, A. Martínez, B. Segura, G. Silva, F. Urra, A. Zapata, M. Penna, O. Acevedo and particularly to J. Constanzo, J. Lagos, F. Norambuena, and J. Troncoso-Palacios for their invaluable help in the field and laboratory. We are very grateful for the language improvements and important comments made by T.F. Hansen, and for the comments made by H. Díaz, C. Reyes-Olivares, F. Toledo and two anonymous reviewers, all of which improved significantly this manuscript. M. Hoare was supported by a fellowship from Fundación Guillermo Puelma (Universidad de Chile). Funds came from Fondecyt 1090251/1120181 (AL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Labra, A., Hoare, M. Chemical recognition in a snake–lizard predator–prey system. acta ethol 18, 173–179 (2015). https://doi.org/10.1007/s10211-014-0203-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10211-014-0203-7