Abstract
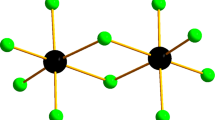
The crystal and molecular structures of [Me2Etim]Cl, [Me2Etim]2[CoCl4], and [Me2Etim]2[NiCl4] ([Me2Etim]+ = 1,2-dimethyl-3-ethylimidazolium cation) all contain evidence that the H4 and H5 protons of the imidazolium cation enter into hydrogen bonds; the implications of this observation for the interactions in room-temperature chloroaluminate(III) ionic liquids are considered.
Similar content being viewed by others
References
Fannin, A. A. Jr.; King, L. A.; Levisky, J. A.; Wilkes, J. S.J. Phys. Chem. 1984,88, 2609.
Tait, S.; Osteryoung, R. A.Inorg. Chem. 1984,23, 4352.
Abdul-Sada, A. K.; Greenway, A. M.; Hitchcock, P. B.; Mohammed, T. J.; Seddon, K. R.; Zora, J. A.J. Chem. Soc., Chem. Commun. 1986, 1753.
Hitchcock, P. B.; Seddon, K. R.; Welton, T.J. Chem. Soc,Dalton Trans. to be submitted.
Wallwork, S. C.Acta Crystallogr. 1962,15, 758.
Pletcher, J.; Sax, M.J. Am Chem. Soc. 1972,94, 3998.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abdul-Sada, A.K., Al-Juaid, S., Greenway, A.M. et al. Upon the hydrogen-bonding ability of the H4 and H5 protons of the imidazolium cation. Struct Chem 1, 391–394 (1990). https://doi.org/10.1007/BF01374488
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01374488