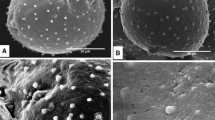
Summary
Ultrastructural details of dry (7% moisture content) and hydratedPyrus communis L. pollen are revealed following freezesubstitution preparation for electron microscopy. Dry pollen is characterized by tightly packed, multilamellate membranous profiles found in association with plasma membrane, vesicles, ER, dictyosomes and some double-membrane bound organelles. Dry pollen also shows unit-membrane bound, densely osmiophilic bodies often with tightly packed multilamellations contained within and, at times, in their bounding membranes. These features are not evident in hydrated pollen. Results suggest that multilamellate membranes form as the plasma membrane, vesicles, ER, and double-membrane bound organelles undergo dehydration, and that upon hydration they rapidly resume normal unilamellate structure.
Similar content being viewed by others
Abbreviations
- DOB:
-
densely osmiophilic body
- IMP:
-
intramembrane particles
- MO:
-
multilamellate organelle
References
Barnabas B (1985) Effect of water loss on germination ability of maize (Zea mays L.) pollen. Ann Bot 55: 201–204
Bliss RD, Platt-Aloia KA, Thomson WW (1984) Changes in plasmalemma organization in cowpea radicle during imbibition in water and NaCl solutions. Plant Cell Environ 7: 601–606
Buttrose MS (1973) Rapid water uptake and structural changes in imbibing seed tissues. Protoplasma 77: 111–122
Chabot JF, Leopold AC (1982) Ultrastructural changes of membranes with hydration in soybean seeds. Am J Bot 69: 623–633
Dowgert MF, Steponkus PL (1984) Behavior of the plasma membrane of isolated protoplasts during a freeze-thaw cycle. Plant Physiol 75: 1139–1151
Dumas C, Gaude T (1981) Stigma pollen recognition and pollen hydration. Phytomorphology 31: 191–201
—, Knox RB, Gaude T (1984) Pollen-pistil recognition: new concepts from electron microscopy and cytochemistry. Int Rev Cytol 90: 239–272
Elleman CJ, Dickinson HG (1986) Pollen-stigma interactions inBrassica. IV. Structural reorganization in the pollen grains during hydration. J Cell Sci 80: 141–157
—, Willison CE, Dickinson HG (1987) Fixation ofBrassica oleracea pollen during hydration: a comparative study. Pollen Spores 29: 273–290
Gilkey JC, Staehelin LA (1986) Advances in ultrarapid freezing for the preservation of cellular ultrastructure. J Electron Microsc Tech 3: 177–210
Gordon-Kamm WJ, Steponkus PL (1984 a) The influence of cold acclimation on the behavior of the plasma membrane following osmotic contraction of isolated protoplasts. Protoplasma 123: 161–173
— — (1984 b) The behavior of the plasma membrane following osmotic contraction of isolated protoplasts: implications in freezing injury. Protoplasma 123: 83–94
Heslop-Harrison J (1979 a) An interpretation of the hydrodynamics of pollen. Am J Bot 66: 737–743
— (1979 b) Aspects of the structure, cytochemistry and germination of the pollen of rye (Secale cereale L.). Ann Bot 44 [Suppl 1]: 1–47
— (1987) Pollen germination and pollen-tube growth. Int Rev Cytol 107: 1–78
Hoekstra FA (1983) Physiological evolution in angiosperm pollen: possible role of pollen vigour. In: Mulcahy DL, Ottaviano E (eds) Pollen biology and implications for plant breeding. Elsevier, New York, pp 35–41
Kerhoas C, Gay G, Dumas C (1987) A multidisciplinary approach to the study of the plasma membrane ofZea mays pollen during controlled dehydration. Planta 171: 1–10
Lancelle SA, Callaham DA, Hepler PK (1986) A method for rapid freeze fixation of plant cells. Protoplasma 131: 153–165
Lin JJ, Dickinson DB (1984) Ability of pollen to germinate prior to anthesis and effect of desiccation on germination. Plant Physiol 74: 746–748
Luza JG, Polito VS (1987) Effects of desiccation and controlled rehydration on germination in vitro of pollen of walnut (Juglans spp.). Plant Cell Environ 10: 487–492
Luzzati V, Husson F (1962) The structure of the liquid-crystalline phases of lipid-water systems. J Cell Biol 12: 207–219
McKersie BD, Stinson RH (1980) Effect of dehydration on leakage and membrane structure inLotus corniculatus L. seeds. Plant Physiol 66: 316–320
Nir I, Klein S, Poljakoff-Mayber A (1969) Effect of moisture stress on submicroscopic structure of maize roots. Aust J Biol Sci 22: 17–33
O'Brien TP, McCully ME (1981) The study of plant structure. Principles and selected methods. Termarcarphi Pvt, Melbourne
Öpik H (1985) The fine structure of some dry seed tissues observed after completely anhydrous chemical fixation. Ann Bot 56: 453–466
Pearce RS (1982) Ultrastructure of tall fescue (Festuca arundinacea Schreb. cv. S 170) cells fixed while exposed to lethal or non-lethal extracellular freezing. New Phytol 92: 259–272
—, Willison JHM (1985) A freeze-etch study of the effects of extracellular freezing on cellular membranes of wheat. Planta 163: 304–316
Platt-Aloia KA, Thomson WW (1985) Freeze-fracture evidence of gel-phase lipids in membranes of senescing cowpea cotyledons. Planta 163: 360–369
—, Lord EM, Demason DA, Thomson WW (1986) Freeze-fracture observations on membranes of dry and hydrated pollen fromCollomia, Phoenix andZea. Planta 168: 291–298
Priestly DA, De Kruijff B (1982) Phospholipid motional characteristic in a dry biological system. A31P-nuclear magnetic resonance study of hydratingTypha latifolia pollen. Plant Physiol 70: 1075–1078
Seewaldt V, Priestly DA, Leopold AC, Feigenson GW, Goodsaid-Zalduondo F (1981) Membrane organization in soybean seeds during hydration. Planta 152: 19–23
Shivanna KR, Heslop-Harrison J (1981) Membrane state and pollen viability. Ann Bot 47: 759–770
Simon EW (1974) Phospholipids and plant membrane permeability. New Phytol 73: 377–420
Singh J (1979) Ultrastructural alterations in cells of hardened and non-hardened winter rye during hyperosmotic and extracellular freezing stresses. Protoplasma 98: 329–341
Steponkus PL (1984) Role of the plasma membrane in freezing injury and cold acclimation. Ann Rev Plant Physiol 35: 543–584
Thomson WW (1979) Ultrastructure of dry seed tissue after a nonaqueous primary fixation. New Phytol 82: 207–212
—, Platt-Aloia K (1982) Ultrastructure and membrane permeability in cowpea seeds. Plant Cell Environ 5: 367–373
Tiwari SC, Polito VS (1988) Spatial and temporal organization of actin during hydration, activation, and germination of pollen inPyrus communis L.: a population study. Protoplasma 147: 5–15
Webb MA, Arnott HJ (1982) Cell wall conformation in dry seeds in relation to the preservation of structural integrity during desiccation. Am J Bot 69: 1657–1668
Webster BD, Leopold AC (1977) The ultrastructure of dry and imbibed cotyledons of soybean. Am J Bot 64: 1286–1293
Yatsu LY (1983) Electron microscopy of dry peanut (Arachis hypogaea L.) seeds crushed for oil removal. Fixation and embedding of anhydrously prepared tissues. Protoplasma 117: 1–6
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tiwari, S.C., Polito, V.S. & Webster, B.D. In dry pear (Pyrus communis L.) pollen, membranes assume a tightly packed multilamellate aspect that disappears rapidly upon hydration. Protoplasma 153, 157–168 (1990). https://doi.org/10.1007/BF01354000
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01354000