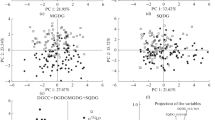
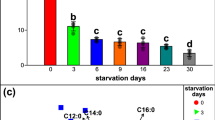
Abstract
We undertook a detailed analysis of the lipid composition ofSolemya velum (Say), a bivalve containing endosymbiotic chemoautotrophic bacteria, in order to determine the presence of lipid biomarkers of endosymbiont activity. The symbiont-free clamMya arenaria (L.) and the sulfur-oxidizing bacteriumThiomicrospira crunogena (Jannasch et al.) were analyzed for comparative purposes. Theδ 13C ratios of the fatty acids and sterols were also measured to elucidate potential carbon sources for the lipids of each bivalve species. Both fatty acid and sterol composition differed markedly between the two bivalves. The lipids ofS. velum were characterized by large amounts of 18: 1ω7 (cis-vaccenic acid), 16:0, and 16 : 1ω7 fatty acids, and low concentrations of the highly unsaturated plant-derived fatty acids characteristic of most marine bivalves. Cholest-5-en-3β-ol (cholesterol) accounted for greater than 95% of the sterols inS. velum. In contrast,M. arenaria had fatty acid and sterol compositions similar to typical marine bivalves and was characterized by large amounts of the highly unsaturated fatty acids 20 : 5ω3 and 22 : 6ω3 and a variety of plant-derived sterols. The fatty acids ofT. crunogena were similar to those ofS. velum and were dominated by 18:1ω7, 16:0 and 16:1ω7 fatty acids. Thecis-vaccenic acid found inS. velum is almost certainly symbiontderived and serves as a potential biomarker for symbiontlipid incorporation by the host. The high concentrations ofcis-vaccenic acid (up to 35% of the total fatty acid content) in both symbiont-containing and symbiont-free tissues ofS. velum demonstrate the importance of the endosymbionts in the lipid metabolism of this bivalve. The presence ofcis-vaccenic acid in all the major lipid classes ofS. velum demonstrates both incorporation and utilization of this compound. Theδ 13C ratios of the fatty acids and sterols ofS. velum were significantly lighter (−38.4 to −45.3‰) than those ofM. arenaria (−23.8 to − 24.2‰) and were similar to the values found for the fatty acids ofT. crunogena (−45‰); this suggests that the lipids ofS. velum are either derived directly from the endosymbionts or are synthesized using endosymbiontderived carbon.
Similar content being viewed by others
Literature cited
Anderson, A. E., Childress, J. J., Favuzzi, J. A. (1987). Net uptake of CO2 driven by sulphide and thiosulphate oxidation in the bacterial symbiont-containing clamSolemya reidi. J. exp. Biol. 133: 1–31
Bobbie, R. J., White, D. C. (1980). Characterization of benthic microbial community structure by high-resolution gas chromatography of fatty acid methyl esters. Appl envirl. Microbiol. 39: 1212–1222
Cavanaugh, C. M. (1983). Symbiosis of chemoautotrophic bacteria and marine invertebrates from sulphide-rich habitats. Nature, Lond. 302: 58–61
Cavanaugh, C. M. (1985). Symbiosis of chemoautotrophic bacteria and marine invertebrates. Ph.D. Thesis, Harvard University
Cavanaugh, C. M., Abbott, M. S., Veenhuis, M. (1988). Immunochemical localization of ribulose-1,5-biphosphate carboxylase in the symbiont-containing gills ofSolemya velum (Bivalvia: Mollusca). Proc. natn. Acad. Sci. USA 85: 7786–7789
Conway, N. (1990). The nutritional role of endosymbiotic bacteria in animal-bacteria symbioses:Solemya velum, a case study. MIT/WHOI Ph.D. thesis
Conway, N., McDowell Capuzzo, J. (1990). The use of biochemical indicators in the study of trophic interactions in animal-bacteria symbioses:Solemya velum, a case study. In: Barnes, M., Gibson, R. N. (eds.) Trophic relationships in the marine environment. Proc. 24th Eur. mar. biol. Symp. Aberdeen University Press, Aberdeen, p. 553–564
Conway, N., McDowell Capuzzo, J., Fry, B. (1989). The role of endosymbiotic bacteria in the nutrition ofSolemya velum: Evidence from a stable isotope analysis of endosymbionts and host. Limnol. Oceanogr. 34: 149–155
DeBurgh, M. E., Juniper, S. K., Singla, C. L. (1989). Bacterial symbiosis in Northeast Pacific Vestimentifera: a TEM study. Mar. Biol 101: 97–105
Degens, E. T. (1969). Biogeochemistry of stable carbon isotopes. In: Eglinton, E., Murphy, M. J. T. (eds.) Organic geochemistry. Springer-Verlag, Berlin, p. 304–329
DeLong, E. F., Yayanos, A. A. (1986). Biochemical function and ecological significance of novel bacterial lipids in deep-sea procaryotes. Appl. envirl Microbiol. 51: 730–737
Fagerland, U. H. M., Idler, D. R. (1960). Marine sterols. VI. Sterol biosynthesis in molluscs and echinoderms. Can. J. Biochem. Physiol. 38: 997–1002
Farrington, J. W., Davis, A. C., Sulanowski, J., McCaffrey, M. A., Clifford, C. H., Dickenson, P., Volkman, J. K. (1988). Biogeochemistry of lipids in surface sediments of the Peru upwelling area — 15°S. In: Mattivelli, L., Novelli, L. (eds.) Advances in organic geochemistry 1987. Org. Geochem. 13: 607–617
Felbeck, H. (1983). Sulfide oxidation and carbon fixation by the gutless clamSolemya reidi: an animal-bacteria symbiosis. J. comp. Physiol. 152: 3–11
Felbeck, H., Childress, J. J., Somero, G. N. (1981). Calvin-Benson cycle and sulfide oxidation enzymes in animals from sulfide-rich habitats. Nature, Lond. 293: 291–293
Fiala-Médioni, A. (1984). Ultrastructural evidence of abundance of intracellular symbiotic bacteria in the gills of bivalve molluscs of deep hydrothermal vents. Cr. Lebd. Séanc. Acad. Sci., Paris (Ser. III) 298: 487–492
Fiala-Médioni, A., Alayse, A. M., Cahet, G. (1986). Evidence of in situ uptake and incorporation of bicarbonate and amino acids by a hydrothermal vent mussel. J. exp. mar. Biol. Ecol. 96: 191–198
Fiala-Médioni, A., LePennec, M. (1987). Trophic structural adaptations in relation to the bacterial association of bivalve molluscs from hydrothermal vents and subduction zones. Symbiosis 4: 63–74
Fulco, A. J. (1983). Fatty acid metabolism in bacteria. Prog. Lipid Res. 22: 133–160
Gagosian, R. B. (1975). Sterols in the western North Atlantic Ocean. Geochim. Cosmochim. Acta 39: 1443–1454
Gardner, D., Riley, J. P. (1972). The component fatty acids of the lipids of some species of marine and freshwater molluscs. J. mar. biol. Ass. U.K. 52: 827–838
Giere, O., Conway, N. M., Gastrock, G., Schmidt, C. (1991). ‘Regulation’ of gutless annelid ecology by endosymbiotic bacteria. Mar. Ecol. Prog. Ser. 68: 287–299
Giere, O., Felbeck, H., Dawson, R., Liebezeit, G. (1984). The gutless marine oligochaetePhallodrilus leukodermatus Giere, a tubificid of structural, ecological and physiological significance. Hydrobiologica 115: 83–89
Gillan, F. T., Johns, R. B. (1986). Chemical markers for marine bacteria: Fatty acids and pigments. In: Johns, R. B. (ed.) Biological markers in the sedimentary environment. Elsevier, Amsterdam, p. 291–309
Gillan, F. T., Stoilov, I. L., Thompson, J. E., Hogg, R. W., Wilkinson, C. R., Djerassi, C. (1988). Fatty acids as biological markers for bacterial symbionts in sponges. Lipids 23: 1139–1145
Goad, L. J. (1976). The steroids of marine algae and invertebrate animals. In: Malins, D. C., Sargent, J. R. (eds.) Biochemical and biophysical perspectives in marine biology, vol. 3. Academic Press, New York, p. 213–318
Goldfine, H. (1972). Comparative aspects of bacterial lipids. Adv. microb. Physiol. 8: 1–58
Guarnieri, M., Johnson, R. M. (1970). The essential fatty acids. Adv. Lipid Res 8: 115–174
Hazel, J. R., Sellner, P. A. (1979). The regulation of membrane lipid composition in thermally acclimated poikilotherms. In: Gilles, R. (ed.) Animals and environmental fitness. Physiological and biochemical aspects of adaptation and ecology. Proc. 1st Conf. Eur. Soc. Comp. Physiol. Biochem. Pergamon Press, New York, p. 541–559
Idler, D. R., Wiseman, P. (1971). Sterols of Molluscs. Int. J. Biochem. 2: 516
Jannasch, H. W., Wirsen, C. O., Nelson, D. C., Robertson, L. A. (1985).Thiomicrospira crunogena sp. nov., a colorless, sulfuroxidizing bacterium from a deep-sea hydrothermal vent. Int. J. system Bact. 35: 422–424
Johns, R. B., Perry, G. J. (1977). Lipids of the marine bacteriumFlexibacter polymorphus. Archs Microbiol. 114: 267–271
Joseph, J. D. (1982). Lipid composition of marine and estuarine invertebrates. Part II: Mollusca. Prog. Lipid Res. 22: 109–153
Katayama-Fujimura, Y., Tsuzaki, N., Kuraishi, H. (1982). Ubiquinone, fatty acid and DNA base composition determination as a guide to the taxonomy of the genus Thiobacillus. J. gen. Microbiol. 128: 1599–1611
Kokko, W. C. M. C., Epstein, S., Look, S. A., Rau, G., Fenical, W., Djerassi, C. (1984). Culture studies and an application of13C/12C isotope ratio mass spectrometry. J. biol. Chem. 259: 8168–8173
McCaffrey, M. A., Farrington, J. W., Repeta, D. J. (1989). Geochemical implications of the lipid composition ofThioploca sp. from the Peru upwelling region — 15°S. Org. Geochem. 14: 61–68
Monson, K. D., Hayes, J. M. (1982). Carbon isotope fractionation in the biosynthesis of bacterial fatty acids. Ozonolysis of unsaturated fatty acids as a means of determining the intramolecular distribution of carbon isotopes. Geochim. Cosmochim. Acta. 46: 139–149
Moreno, J., Pollero, A. E., Moreno, V. J., Brenner, R. R. (1980). Lipids and fatty acids of the mussel (Mytilus platensis d'Orbigny) from South Atlantic Waters. J. exp. mar. Biol. Ecol. 48: 263–276
Okuyama, H., Yamada, K., Kameyama, Y., Ikezawa, H., Akamatzu, Y., Nojima, S. (1977). Regulation of membrane lipid synthesis inEscherichia coli after shifts in temperature. Biochem. 16: 2668–2673
Ourisson, G., Albrecht, P., Rohmer, M. (1979). The hopanoids. Palaeochemistry and biochemistry of a group of natural products. Pure appl. Chem. 51: 709–729
Parkes, R. J., Taylor, J. (1983). The relationship between fatty acid distributions and bacterial respiratory types in contemporary marine sediments. Estuar. cstl mar. Sci. 16: 173–189
Perry, G. J., Volkman, J. K., Johns, R. B, Bavor, H. J. Jr. (1979). Fatty acids of bacterial origin in contemporary marine sediments. Geochim. Cosmochim. Acta 43: 1715–1725
Phillips, N. W. (1984). The role of different microbes and substrates as potential suppliers of specific, essential nutrients to marine detritivores. Bull. mar. Sci. 35: 283–298
Piretti, M. V., Pistore, R., Pagliuca, G. (1989). Uptake and utilization of lipid constituents dispersed in culture water by the bivalve molluscScapharca inaequivalvis (Bruguiere). Comp. Biochem. Physiol. 92B: 755–758
Piretti, M. V., Tioli, F., Pagliuca, G. (1987). Investigation of the seasonal variations of sterol and fatty acid constituents in the bivalve molluscsVenus gallina andScapharca inaequivalvis (Bruguiére). Comp. Biochem. Physiol. 88B: 1201–1208
Prahl, F. G., Meuhlhausen, L. A. (1989). Lipid biomarkers as geochemical tools for paleoceanographic study. Berger, W. H., Smetacek, V. S., Wefer, G. (eds.) Productivity of the ocean: present and past. T. Wiley and Sons limited, New York, p. 271–289
Rau, G. H. (1985).13C/12C and15N/14N in hydrothermal vent organisms: ecological and biogeochemical implications. Bull. biol. Soc. Wash. 6: 243–247
Ruby, E. G., Jannasch, H. W., Deuser, W. G. (1987). Fractionation of stable carbon isotopes during chemoautotrophic growth of sulfur-oxidizing bacteria. Appl. envirl. Microbiol. 53: 1940–1943
Southward, A. J., Southward, E. C., Dando, P. R., Rau, G. H., Fehlbeck, H., Flugel, H. (1981). Bacterial symbionts and low13C/12C ratios in tissues of Pogonophora suggest unusual nutrition and metabolism. Nature, Lond. 293: 616–620
Teshima, S.-I., Kanazawa, A. (1974). Biosynthesis of sterols in abalone,Haliotis gurneri, and mussel,Mytilus edulis. Comp. Biochem. Physiol 47B: 555–561
Teshima, S.-I., Patterson, S. W. (1981). Sterol biosynthesis in the oysterCrassostrea virginica. Lipids 16: 234–239
Volkman, J. K. (1986). A review of sterol markers for marine and terrigenous organic matter. Org. Geochem 9: 83–99
Volkman, J. K., Johns, R. B., Gillan, F. T., Perry, G. J., Bavor, H. J. Jr (1980). Microbial lipids of an intertidal sediment. Fatty acids and hydrocarbons. Geochem. Cosmochim. Acta 44: 1133–1143
Voogt, P. A. (1975). Investigations of the capacity of synthesizingβ-sterols in Mollusca. XII. Biosynthesis and composition of sterols in some bivalves (Anisomyasia). Comp. Biochem. Physiol. 50B: 499–504
Voogt, P. A. (1972). Lipid and sterol components and metabolism in Mollusca. In: Florkin, M., Sheer, B. T. (eds.) Chemical zoology, Vol. 7. Academic Press, London and New York, p. 245
Wada, M., Fukunaga, N., Sasaki, S. (1989). Mechanism of biosynthesis of unsaturated fatty acids inPseudomonas sp. strain E-3, a psychotrophic bacterium. J. Bacteriol. 171: 4267–4271
Author information
Authors and Affiliations
Additional information
Communicated by J. Grassle, Woods Hole
Woods Hole Oceanographic Institution Contribution No. 7356
Please address all correspondence and reprint requests to Dr Conway at her present address: Department of Biological Sciences, University of Pittsburgh, Pennsylvania 15260, USA
Rights and permissions
About this article
Cite this article
Conway, N., McDowell Capuzzo, J. Incorporation and utilization of bacterial lipids in theSolemya velum symbiosis. Mar. Biol. 108, 277–291 (1991). https://doi.org/10.1007/BF01344343
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01344343