Summary
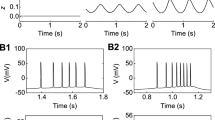
Evidence suggests that the prefrontal cortex (PFC) plays an important role in the burst activity of midbrain dopaminergic (DA) neurons. In particular, electrical stimulation of the PFC elicits patterns of activity in DA neurons, closely time-locked to the stimulation, which resemble natural bursts. Given that natural bursts are produced by the activity of excitatory amino acid (EAA)-ergic afferents, if PFC-induced time-locked bursts are homologues of natural bursts, EAA antagonists should attenuate them. Hence, the NMDA (N-methy1-D-aspartate) antagonist CPP (3-((±)-2-carboxypiperazin-4-yl)propyl-1-phosphonic acid) and the AMPA (D,L-α-amino-3-hydroxy-5-methyl-4-isoxalone propionic acid)/kainate antagonist CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) were applied by iontophoresis to DA neurons exhibiting time-locked bursts during PFC stimulation. CPP produced a significant reduction in time-locked bursting. In contrast, CNQX (at currents which antagonised AMPA responses) did not. These effects of CPP and CNQX on time-locked bursting mirror the effects previously reported for these drugs on natural bursting. Since natural bursting and bursting induced by PFC stimulation are both blocked selectively by CPP, the present results increase the degree of analogy between the two burst phenomena, thereby adding extra support to the contention that the cortex is involved in producing the natural bursting in DA neurons.
Similar content being viewed by others
References
Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL (1991) Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature 6305: 156–158
Bliss TVP, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39
Carter CJ (1982) Topograghical distribution of possible glutamatergic pathways from the frontal cortex to the striatum and substantia nigra in rats. Neuropharmacology 21: 379–383
Chergui K, Charlety PJ, Akaola H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G (1993) Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neuronsin vivo. Eur J Neurosci 5: 137–144
Christie MJ, Bridge S, James LB, Beart PM (1985) Excitotoxin lesions suggest an aspartatergic projection from the medial prefrontal cortex to the ventral tegmental area. Brain Res 333: 169–172
Clark D, Chiodo LA (1988) Electrophysiological and pharmacological characterisation of identified nigrostriatal and mesoaccumbens dopamine neurons in the rat. Synapse 2: 474–485
Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ (1996) Drugs of abuse and stress incease the expression of GluR1 and NMDAR1 glutamate receptor subunits in the ventral tegmental area: common adaptations among cross-sensitising agents. J Neurosci 16: 274–282
Freeman AS, Meltzer LT, Bunney BS (1985) Firing properties of substantia nigra dopaminergic neurons in freely moving rats. Life Sci 36: 1983–1994
Gariano RF, Groves PM (1988) Burst firing in midbrain dopamine neurons by stimulation of the medial prefrontal and anterior cingulate cortices. Brain Res 462: 194–198
Gonon FG (1988) Nonlinear relationship between impulse flow and dopamine released by rat midbrain neurons as studied byin vivo electrochemistry. Neuroscience 24: 19–28
Grace AA, Bunney BS (1983) Intracellular and extracellular electrophysiology of nigral dopaminergic neurons. 1. Identification and characterisation. Neuroscience 10: 301–315
Grace AA, Bunney BS (1984a) The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 4: 2866–2876
Grace AA, Bunney BS (1984b) The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4: 2877–2890
Grace AA, Onn S-P (1989) Morphology and electrophysiological properties of immuno-cytochemically identified rat dopamine neurons recordedin vitro. J Neurosci 9: 3463–3481
Grenhoff J, Ugedo L, Svensson TH (1988) Firing patterns of midbrain dopamine neurons: differences between A9 and A10. Acta Physiol Scand 134: 127–132
Ip NY, Zigmond RE (1984) Pattern of presynaptic nerve activity can determine the type of neurotransmitter regulating a postsynaptic event. Nature 311: 472–474
Kalivas PW, Alesdatter JE (1993) Involvement ofN-methyl-D-aspartate receptor stimulation in the ventral tegmental area and the amygdala in behavioural sensitization to cocaine. J Pharmacol Exp Ther 267: 486–495
Kang Y, Kitai ST (1993a) Calcium spike underlying rhythmic firing in dopaminergic neurons of the rat substantia nigra. Neurosci Res 18: 195–207
Kang Y, Kitai ST (1993b) A whole cell patch-clamp study on the pacemaker potential in dopaminergic neurons of rat substantia nigra compacta. Neurosci Res 18: 209–221
Karreman M, Moghaddam B (1996) The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem 66: 589–598
Kornhuber J, Kim J-S, Kornhuber ME, Kornhuber HH (1984) The cortico-nigral projection: reduced glutamate content in the substantia nigra following frontal cortex ablation in the rat. Brain Res 322: 124–126
Mercuri NB, Stratta F, Calabresi P, Bonchi A, Bernardi G (1993) Activation of metabotropic glutamate receptors induces an inward current in rat dopamine mesencephalic neurons. Neuroscience 56: 399–407
Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH (1993) Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studiedin vivo. Neurosci Lett 157: 53–56
Overton P, Clark D (1992) Iontophoretically administered drugs acting at theN-methyl-D-aspartate receptor modulate burst firing in A9 dopamine neurons in the rat. Synapse 10: 131–140
Overton PG, Tong Z-Y, Clark D (1996) A pharmacological analysis of the burst events induced in midbrain dopaminergic neurons by electrical stimulation of the prefrontal cortex in the rat. J Neural Transm [Gen Sect] 103: 523–540
Robinson TE (1993) Persistent sensitizing effects of drugs on brain dopamine systems and behaviour: implications for addiction and relapse. In: Korenman SG, Barchas JD (eds) Biological basis of substance abuse. Oxford University Press, New York, pp 373–402
Schoepp D, Bockaert J, Sladeczek F (1990) Pharmacology and functional characteristics of metabotropic excitatory amino acid receptors. Trends Pharmacol Sci 11: 508–515
Seutin V, Verbanck P, Massotte L, Dresse A (1990) Evidence for the presence ofN-methyl-D-aspartate receptors in the ventral tegmental area of the rat: an electrophysiological in vitro study. Brain Res 514: 147–150
Silva NL, Bunney BS (1988) Intracellular studies of dopamine neuronsin vitro: pacemakers modulated by dopamine. Eur J Pharmacol 149: 307–315
Stone TW (1985) Microiontophoresis and pressure ejection. Methods in the neurosciences, vol 8. Wiley, Chichester (IBRO handbook series)
Svensson TH, Tung C-S (1989) Local cooling of the prefrontal cortex induces pacemaker-like firing of dopamine neurons in rat ventral tegmental areain vivo. Acta Physiol Scand 136: 135–136
Taber MT, Das S, Fibiger HC (1995) Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J Neurochem 65: 1407–1410
Tepper JM, Sawyer SF, Groves PM (1987) Electrophysiologically identified dopaminergic neurons intracellularly labeled with HRP: light microscopic analysis. J Neurosci 7: 2794–2806
Tong Z-Y, Overton PG, Clark D (1995) Chronic administration of (+)-amphetamine alters the reactivity of midbrain dopaminergic neurons to prefrontal cortex stimulation in the rat. Brain Res 674: 63–74
Tong Z-Y, Overton PG, Clark D (1996) Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse 22: 195–208
Wang RY (1981) Dopaminergic neurons in the rat ventral tegmental area. I. Identification and characterisation. Brain Res Rev 3: 123–140
White FJ, Hu X-T, Zhang X-F, Wolf ME (1995) Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther 273: 445–454
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tong, Z.Y., Overton, P.G. & Clark, D. Antagonism of NMDA receptors but not AMPA/kainate receptors blocks bursting in dopaminergic neurons induced by electrical stimulation of the prefrontal cortex. J. Neural Transmission 103, 889–904 (1996). https://doi.org/10.1007/BF01291780
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01291780