Summary
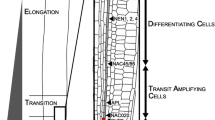
Cell development in the root apical meristem is thought to be regulated by position-dependent information, but as yet, the underlying mechanism for this remains unknown. In order to examine the potential involvement of the symplasmic transmission of positional signals, plasmodesmatal frequency and distribution was quantitatively analyzed in root apical meristem cell walls ofArabidopsis thaliana during root development. A consistent distribution pattern of plasmodesmata was observed in the root apex over four weeks. While cells within initial tiers were uniformly interconnected, more symplasmic connections between the initial tiers and their immature-cell (primary-meristem) derivatives were observed than within the initial tiers. Immature cells were connected across transverse walls by primary plasmodesmata according to a tissue-specific pattern. Cells of the immature vascular tissue and cortex had the highest plasmodesmatal frequencies, followed by the immature epidermis and root cap. Although the numbers of plasmodesmata in transverse walls (primary plasmodesmata) was reduced in all tissues as the root aged, the tissue-specific distribution remained constant. The extent of symplasmic coupling across the boundaries of each tissue appeared to be limited by fewer secondary plasmodesmata in longitudinal walls. The frequency of all plasmodesmata decreased as the root aged. The primary plasmodesmata within each tissue increased at one week and then dramatically decreased with root age; the frequency of secondary plasmodesmata in longitudinal walls also decreased, but more gradually. These findings are discussed with respect to the roles likely played by plasmodesmata in facilitating transport of position-dependent information during root development.
Similar content being viewed by others
References
Balachandran S, Hull RJ, Vaadia Y, Wolf S, Lucas WJ (1995) Tobacco mosaic virus movement protein-induced change in carbon partitioning originates from the mesophyll and is independent of change in plasmodesmatal size exclusion limit. Plant Cell Environ 18: 1301–1310
—, Xiang Y, Schobert C, Thompson GA, Lucas WJ (1997) Phloem sap proteins ofCucurbita maxima andRicinus communis have the capacity to traffic cell to cell through plasmodesmata. Proc Natl Acad Sci USA 94: 14150–14155
Barlow PW (1984) Positional controls in root development. In: Barlow PW, Carr DJ (eds) Positional controls in plant development. Cambridge University Press, Cambridge, pp 281–318
—, Carr DJ (eds) (1984) Positional controls in plant development. Cambridge University Press, Cambridge
Baum SF (1996) The developmental organization of the root apical meristem inArabidopsis thaliana cv. WS. PhD dissertation, University of California, Davis, California
—, Rost TL (1996) Root apical organization inArabidopsis thaliana: the root cap and protoderm. Protoplasma 192: 178–188
Carpenter R, Coen ES (1995) Transposon induced chimeras shows thatfloricaula, a meristem identity gene, acts non-autonomously between cell layers. Development 121: 19–26
Ding B, Lucas WJ (1996) Secondary plasmodesmata: biogenesis, special function and evolution. In: Smallwood M, Knox JP, Bowles DJ (eds) Membranes: specialized functions in plant cells. Bios Scientific Publishers, Oxford, pp 489–506
—, Haudenshield JS, Hull RJ, Wolf S, Beachy RN, Lucas WJ (1992) Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in transgenic plants. Plant Cell 4: 915–928
Doerner P (1993) Patterning theArabidopsis root. Curr Biol 3: 867–869
Dolan L (1996) Pattern in the root epidermis: an interplay of diffusible signals and cellular geometry. Ann Bot 77: 547–553
—, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B (1993) Cellular organization of theArabidopsis thaliana root tip. Development 119: 71–84
Duckett CM, Oparka KJ, Prior DAM, Dolan L, Roberts K (1994) Dye-coupling in the root epidermis ofArabidopsis is progressively reduced during development. Development 120: 3247–3255
Erwee MG, Goodwin PB (1985) Symplast domains in extrastellar tissues ofEgeria densa Planch. Planta 163: 9–19
Fisher DB, Wu Y, Ku MSB (1992) Turnover of soluble proteins in the wheat sieve tube. Plant Physiol 100: 1433–1441
Galway M, Masucci J, Lloyd A, Walbot V, Davis R, Schiefelbein J (1994) TheTTG gene is required to specify epidermal cell fate and cell patterning in theArabidopsis root. Dev Biol 166: 740–754
Gladish DK, Rost TL (1993) The effects of temperature on primary root growth dynamics and lateral root distribution in garden pea (Pisumsativum L., cv. “Alaska”). Environ Exp Bot 33: 243–258
Gunning BES (1978) Age-related and origin-related control of the number of plasmodesmata in cell walls of developingAzolla roots. Planta 143: 181–190
—, Robards AW (eds) (1976) Intercellular communication in plants: studies on plasmodesmata. Springer, Berlin Heidelberg New York
Hake S, Char BR (1997) Cell-cell interactions during plant development. Genes Dev 11: 1087–1097
Hantke SS, Carpenter R, Coen ES (1995) Expression offloricaula in single layers of periclinal chimeras activates downstream homeotic genes in all layers of floral meristems. Development 121: 27–35
Jackson D, Hake S (1997) Morphogenesis on the move: cell-to-cell trafficking of plant regulatory proteins. Curr Opin Genet Dev 7: 495–500
—, Veit B, Hake S (1994) Expression of maize KNOTTED 1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120: 405–413
Kragler F, Lucas WJ, Monzer J (1998) Plasmodesmata: dynamics, domains and patterning. Ann Bot 81: 1–10
Kwiatkowska M (1988) Symplasmic isolation ofChara vulgaris antheridium and mechanisms regulating the process of spermatogenesis. Protoplasma 142: 137–146
Lawrence PA, Struh G (1996) Morphogens, compartments, and patterning: lesson fromDrosophila? Cell 85: 951–961
Lucas WJ (1995) Plasmodesmata: intercellular channels for macromolecular transport in plants. Curr Opin Cell Biol 7: 673–680
—, Ding B, van der School C (1993) Plasmodesmata and the supra-cellular nature of plants. New Physiol 125: 435–476
—, Bouche-Pillon S, Jackson DP, Nguyen L, Baker L, Ding B, Hake S (1995) Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science 270: 1980–1983
—, Balachandran S, Park J, Wolf S (1996) Plasmodesmatal companion cell-mesophyll communication in the control over carbon metabolism and phloem transport: insights gained from viral movement proteins. J Exp Bot 47: 1119–1128
Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW (1996) The homeobox geneGLABRA 2 is required for position-dependent cell differentiation in the root epidermis ofArabidopsis thaliana. Development 122: 1253–1260
Mezitt LA, Lucas WJ (1996) Plasmodesmatal cell-to-cell transport of proteins and nucleic acids. Plant Mol Biol 32: 251–273
Oparka KJ (1993) Signalling via plasmodesmata: the neglected pathway. Sem Cell Biol 4: 131–138
—, Duckett CM, Prior DAM, Fisher DB (1994) Real-time imaging of phloem unloading in the root tip ofArabidopsis. Plant J 6: 759–766
Reinhardt DH, Rost TL (1995) On the correlation of primary root growth and tracheary element size and distribution from the tip in cotton seedlings grown under salinity. Environ Exp Bot 35: 575–588
Robards AW, Lucas WJ (1990) Plasmodesmata. Annu Rev Plant Physiol Plant Mol Biol 41: 369–419
Rost TL (1994) Root tip organization and the spatial relationships of differentiation events. In: Iqbal M (ed) Growth patterns in vascular plants. Dioscordes Press, Portland, pp 59–76
—, Baum S (1988) On the correlation of primary root length, meristem size and protoxylem tracheary element position in pea seedlings. Am J Bot 75: 414–424
—, Bryant JA (1996) Root organisation and gene expression patterns. J Exp Bot 47: 1613–1628
—, Jones TJ, Falk RH (1988) Distribution and relationship of cell division and maturation events inPisum sativum (Fabaceae) seedling roots. Am J Bot 75: 1571–1583
—, Baum SF, Nichol S (1996) Root apical organization inArabidopsis thaliana ecotype “WS” and a comment on root cap structure. Plant Soil 187: 91–95
Scheres B (1997) Cell signaling in root development. Curr Opin Genet Dev 7: 501–506
—, Wolkenfelt H, Willemsen V, Terlouw M, Lawson E, Dean C, Weisbeek P (1994) Embryonic origin of theArabidopsis primary root and root meristem initials. Development 120: 2475–2487
Seagull RW (1983) Differences in the frequency and disposition of plasmodesmata resulting from root cell elongation. Planta 159: 497–504
Spurr AR (1969) A new low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26: 31–43
Tilney LG, Cooke TJ, Connelly PS, Tilney MS (1990) The distribution of plasmodesmata and its relationship to morphogenesis in fern gametophytes. Development 110: 1209–1221
van den Berg C, Willemsen V, Hage W, Scheres B (1995) Determination of cell fate in theArabidopsis root meristem by directional signalling. Nature 378: 62–65
—, Hendriks G, Weisbeek P, Scheres B (1997) Short-range control of cell differentiation in theArabidopsis root meristem. Nature 390: 287–289
van der Schoot, Lucas WJ (1993) Microinjection and the study of tissue patterning in plant apices. In: Maliga P, Klessig D, Cashmore A, Gruissein W, Varner J (eds) Methods in plant molecular biology. Cold Spring Harbor Press, Cold Spring Harbor, NY, pp 173–189
Young SJ, Royer SM, Groves PM, Kinnamon JC (1987) Three-dimensional reconstructions from serial micrographs using the IBM PC. J Electron Microsc Tech 6: 207–218
Zhu T, Lucas WJ, Rost TL (1998) Directional cell-to-cell communication in theArabidopsis apical meristem I: an ultrastructural and functional analysis. Protoplasma 203: 35–47
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhu, T., O'Quinn, R.L., Lucas, W.J. et al. Directional cell-to-cell communication in theArabidopsis root apical meristem II. Dynamics of plasmodesmatal formation. Protoplasma 204, 84–93 (1998). https://doi.org/10.1007/BF01282296
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01282296