Summary
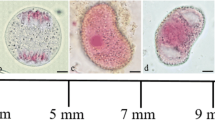
Changes in the number and distribution of mitochondria in microspores and pollen grains during male gametogenesis inPharbitis nil were examined with Technovit sections stained with 3,3′-dihexyloxacarbocyanine iodide. The number of mitochondria per microspore or pollen grain ofP. nil increased constantly and dramatically during male gametogenesis. During this process, mitochondria exhibited characteristic localizations: subpopulations of mitochondria covered the surface of the microspore and vegetative nuclei before and again just after postmeiotic mitosis I (9 and 5 days before flowering, respectively). The mitochondria also surrounded the generative nucleus 2 days after postmeiotic mitosis I (5 days before flowering), although the density of mitochondria on the nuclear surface was lower. Electron microscopy showed that the mitochondria were about 30 nm from the nuclear envelope and that each mitochondrion was located near a nuclear pore. The characteristic localization of mitochondria inP. nil pollen may serve as a model to analyze the mechanisms that control mitochondrial positioning within a cell and interactions between mitochondria and nuclei.
Similar content being viewed by others
Abbreviations
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DiOC6 :
-
3,3′-dihexyloxacarbocyanine iodide
- PM I:
-
postmeiotic mitosis I
References
Bednara J, Gielwanowska I, Rodkiewicz B (1986) Regular arrangements of mitochondria and plastids during sporogenesis inEquisetum. Protoplasma 130: 145–152
Dickinson HG (1981) The structure and chemistry of plastid differentiation during male meiosis inLillian henryi. J Cell Sci 52: 223–241
—, Li FL (1988) Organelle behaviour during higher plant gametogenesis. In: Boffey SA, Lloyd D (eds) The division and segregation of organelles. Cambridge University Press. Cambridge, pp 131–148
—, Potter U (1979) Post-meiotic nucleo-cytoplasmic interaction inCosmos bipinnatus. Planta 145: 449–457
Hagemann R, Schröder MB (1989) The cytological basis of the plastid inheritance in angiosperms. Protoplasma 152: 57–64
Hu S, Zhu C, Xu L, Shen J (1979) Ultrastructure of male gametophyte in wheat I: the formation of generative and vegetative cells. Acta Bot Sin 21: 208–214
Nagata N, Saito C, Sakai A, Kuroiwa H, Kuroiwa T (1999a) Decrease in mitochondrial DNA and concurrent increase in plastid DNA in generative cells ofPharbitis nil during pollen development. Eur J Cell Biol 78: 241–248
— — — — — (1999b) The selective increase or decrease of organellar DNA in generative cells just after pollen mitosis one controls cytoplasmic inheritance. Planta 209: 53–65
Owens JN, Morris SJ (1990) Cytological basis for cytoplasmic inheritance inPseudotsuga menziesii I: pollen tube and archegonial development. Am J Bot 77: 433–445
Pacini E, Taylor PE, Singh MB, Knox RB (1992) Development of plastids in pollen and tapetum of rye-grass,Lolium perenne L. Ann Bot 70: 179–188
Reynolds TL, Raghavan V (1982) An autoradiographic study of RNA synthesis during maturation and germination of pollen grains ofHyoscyamus niger. Protoplasma 111: 177–188
Rodkiewicz B, Bednara J, Mostowska A, Duda E, Stobiecka H (1986) The change in disposition of plastids and mitochondria during microsporogenesis and sporogenesis in some higher plants. Acta Bot Neerl 35: 209–215
Russell SD, Strout GW, Stramski AK, Mislan TW, Thompson RA, Schoemann LM (1996) Microgametogenesis inPlumbago zeylanica (Plumbaginaceae) 1: descriptive cytology and three-dimensional organization. Am J Bot 83: 1435–1453
Saito C, Fujie M, Sakai A, Nagata N, Matsunaga S, Kuroiwa H, Kuroiwa T (1998) A high density of rRNA in the generative cells and sperm cells of pollen grains of five angiosperm species. Cytologia 63: 293–300
Schröder MB, Oldenburg H (1990) Ultrastructural studies on plastids of generative and vegetative cells inLiliaceae 7: plastid distribution during generative cell development inTulbaghia violacea Harv. Flora 184: 131–136
Sodmergen, Luo YY, Kuroiwa T, Hu SY (1994) Cytoplasmic DNA apportionment and plastid differentiation during male gametophyte development inPelargonium zonale. Sex Plant Reprod 7: 51–56
—, Chen GH, Hu ZM, Guo FL, Guan XL (1995) Male gametophyte development inPlumbago zeylanica: cytoplasm localization and cell determination in the early generative cell. Protoplasma 186: 79–86
Tanaka I (1991) Microtuble-determined plastid distribution during microsporogenesis inLilium longiflorum. J Cell Sci 99: 21–31
— (1997) Differentiation of generative and vegetative cells in angiosperm pollen. Sex Plant Reprod 10: 1–7
Terasaki M, Song J, Wong JR, Weiss MJ, Chen LB (1984) Localization of endoplasmic reticulum in living and glutaraldehyde-fixed cells with fluorescent dyes. Cell 38: 101–108
Wagner VT, Cresti M, Salvatici P, Tiezzi A (1990) Changes in volume, surface area, and frequency of nuclear pores on the vegetative nucleus of tobacco pollen in fresh, hydrated, and activated conditions. Planta 181: 304–309
Xu H, Swoboda I, Bhalla PL, Singh MB (1999a) Male gametic cell-specific gene expression in flowering plants. Proc Natl Acad Sci USA 96: 2554–2558
— — — — (1999b) Male gametic cell-specific expression of H2A and H3 histone genes. Plant Mol Biol 39: 607–614
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagata, N., Saito, C., Sakai, A. et al. Unique positioning of mitochondria in developing microspores and pollen grains inPharbitis nil: mitochondria cover the nuclear surface at specific developmental stages. Protoplasma 213, 74–82 (2000). https://doi.org/10.1007/BF01280507
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01280507